|
Phosphorane
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. Phosphoranes have the general formula PR5. Phosphoranes of the type PX5 adopt a trigonal bipyramidal molecular geometry with the two apical bonds longer than the three equatorial bonds. Hypervalent bonding is described by inclusion of non-bonding MOs, as also invoked for the closely related molecule phosphorus pentafluoride. Examples The parent hydride compound is the hypothetical molecule PH5. Pentaphenylphosphorane (Ph5P) is stable. Pentaalkoxyphosphoranes are more common with electronegative substituents. Examples of P(OR)5 (R = alkyl), have however been prepared by reaction of phosphites with benzene alkyl sulfenates: : Wittig reagents Phosphoranes of the type R3P=CR2 are more common and more important. Phosphoranes are also considered to be one of the resonance structures of ylides, these compounds feature a tetrahedral phosphorus center including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
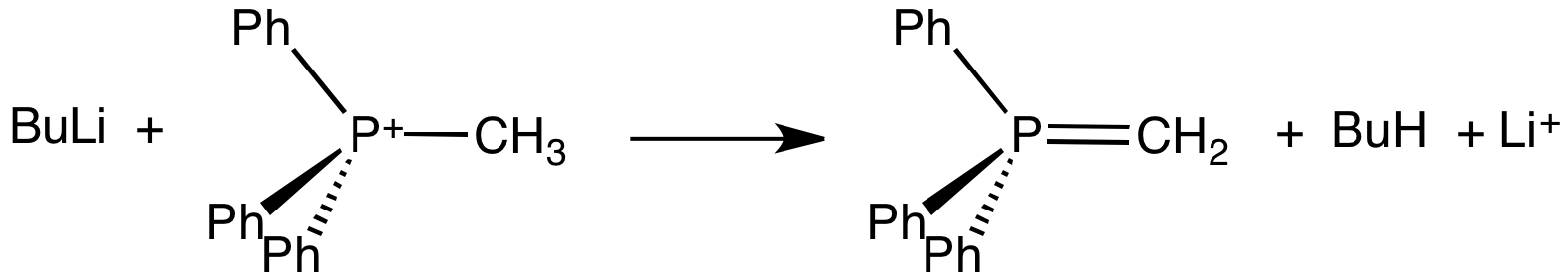
Methylenetriphenylphosphorane
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species. Preparation and use Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium: :Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH The phosphorane is generally not isolated, instead it is used in situ. The estimated pKa of this carbon acid is near 15. Potassium tert-butoxide has been used in place of Butyllithium, butyl lithium. Sodium amide has also been used a base. Methylenetriphenylphosphorane is used to replace oxygen centres in Aldehyde, aldehydes and Ketone, ketones with a methylene group, i.e., a methylenation: :R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO The phosphorus-containing product is triphenylphosphine oxide. Structure Crystallographic characterization of the colourless ylide reveals th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorane
A phosphorane (IUPAC name: λ5-phosphane) is a functional group in organophosphorus chemistry with pentavalent phosphorus. Phosphoranes have the general formula PR5. Phosphoranes of the type PX5 adopt a trigonal bipyramidal molecular geometry with the two apical bonds longer than the three equatorial bonds. Hypervalent bonding is described by inclusion of non-bonding MOs, as also invoked for the closely related molecule phosphorus pentafluoride. Examples The parent hydride compound is the hypothetical molecule PH5. Pentaphenylphosphorane (Ph5P) is stable. Pentaalkoxyphosphoranes are more common with electronegative substituents. Examples of P(OR)5 (R = alkyl), have however been prepared by reaction of phosphites with benzene alkyl sulfenates: : Wittig reagents Phosphoranes of the type R3P=CR2 are more common and more important. Phosphoranes are also considered to be one of the resonance structures of ylides, these compounds feature a tetrahedral phosphorus center including ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypervalent Molecule
In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group elements apparently bearing more than eight electrons in their valence shells. Phosphorus pentachloride (), sulfur hexafluoride (), chlorine trifluoride (), the chlorite () ion in chlorous acid and the triiodide () ion are examples of hypervalent molecules. Definitions and nomenclature Hypervalent molecules were first formally defined by Jeremy I. Musher in 1969 as molecules having central atoms of group 15–18 in any valence other than the lowest (i.e. 3, 2, 1, 0 for Groups 15, 16, 17, 18 respectively, based on the octet rule). Several specific classes of hypervalent molecules exist: * Hypervalent iodine compounds are useful reagents in organic chemistry (e.g. Dess–Martin periodinane) * Tetra-, penta- and hexavalent phosphorus, silicon, and sulfur compounds (e.g. PCl5, PF5, SF6, sulfuranes and persulfuranes) * Nob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Chemistry
Organophosphorus chemistry is the scientific study of the synthesis and properties of organophosphorus compounds, which are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt a variety of oxidation states, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig Reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative. Reaction mechanism Mechanistic studies have focused on unstabilized ylides, because the intermediates can be followed by NMR spectroscopy. The existence and interconversion of the betaine (3a and 3b) is subject of ongoing research. For lithium-free Wittig reactions, studies support a concerted formation of the oxaphosphetane without intervention of a betaine. In particular, phosphonium ylides 1 react with carbonyl compounds 2 via a +2cycloaddition that is sometimes described as having π2s+π2a">sub>π2s+π2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
An ylide () or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2- dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiply bonded form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attractio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentafluoride
Phosphorus pentafluoride is a chemical compound with the chemical formula . It is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Preparation Phosphorus pentafluoride was first prepared in 1876 by the fluorination of phosphorus pentachloride using arsenic trifluoride, which remains a favored method: : Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine: : Structure Single-crystal X-ray studies indicate that the has trigonal bipyramidal geometry. Thus it has two distinct types of P−F bonds (axial and equatorial): the length of an axial P−F bond is distinct from the equatorial P−F bond in the solid phase, but not the liquid or gas phases due to Berry pseudo rotation. Fluorine-19 NMR spectroscopy, even at temperatures as low as , fails to distinguish the axial from the equatorial fluorine environments. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism, by which th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentaphenylphosphorane
Pentaphenylphosphorus is an organic phosphorane containing five phenyl groups connected to a central phosphorus atom. The phosphorus atom is considered to be in the +5 oxidation state. The chemical formula could be written as P(C6H5)5 or Ph5P, where Ph represents the phenyl group. It was discovered and reported in 1949 by Georg Wittig. Formation and history Pentaphenylphosphorus can be formed by the action of phenyllithium on tetraphenylphosphonium bromide or tetraphenylphosphonium iodide. The compound was produced during the course of Wittig's Nobel-prize-winning investigations of organophosphorus compounds. Structure Pentaphenylphosphorus is trigonal bipyramidal, according to several determinations by X-ray crystallography. The axial and equatorial P-C bond lengths are 199 and 185 picometers, respectively. The monoclinic crystal has dimensions a=10.03, b=17.22 c=14.17 Å and β=112.0°. Pentaphenyl phosphorus can also crystallise with solvent, (to form a solvate) with tetr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfenate
In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula . It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids () and sulfonic acids (), respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide. Properties In contrast to sulfinic and sulfonic acids, simple sulfenic acids, such as methanesulfenic acid, CH3SOH, are highly reactive and cannot be isolated in solution. In the gas phase the lifetime of methanesulfenic acid is about one minute. The gas phase structure of methanesulfenic acid was found by microwave spectroscopy (rotational spectroscopy) to be CH3–S–O–H. Sulfenic acids can be stabilized through steric effects, which prevent the sulfenic acid from condensing with itself to form thiosulfinates, RS(O)SR, such as allicin from garlic. Through the use of X-ray crystallography, the structure of such stabilized sulfenic acids were shown to be R–S–O–H. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphane
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air (pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisier ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |