|
Perfluorononanoic Acid
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also a persistent organic pollutant. Chemistry and properties In acidic form it is a highly reactive strong acid. In its conjugate base form as a salt it is stable and commonly ion paired with ammonium. In the commercial product Surflon S-111 (CAS 72968-3-88) it is the primary compound present by weight. PFNA is used as surfactant for the production of the fluoropolymer polyvinylidene fluoride.Supporting Information (PDF) It is produced mainly in Japan by the of a linear |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olefin
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, distinguished by the position and conformation of the double bond. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
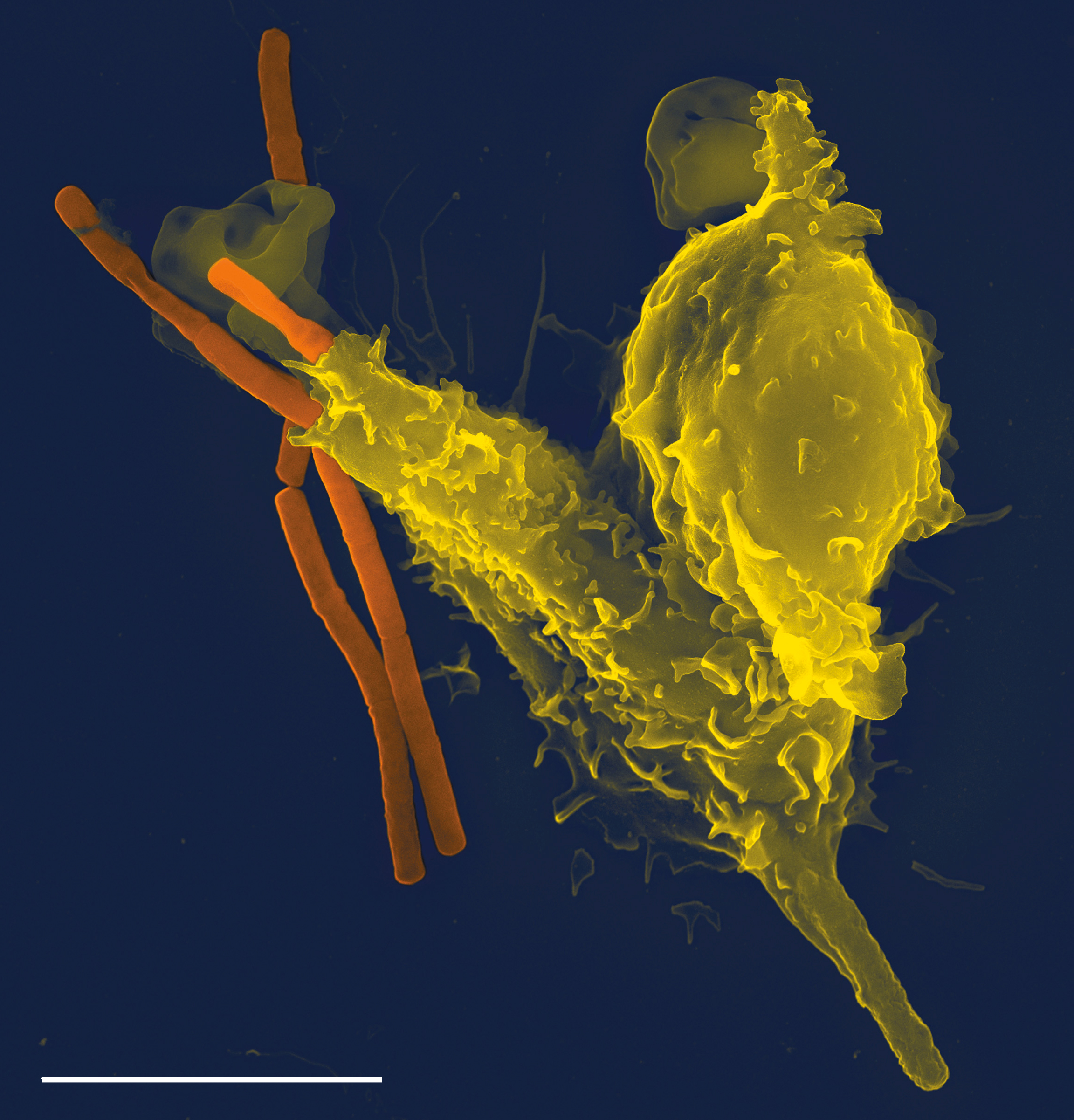
Immune System
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy biological tissue, tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use humoral immunity, molecules and cell-mediated immunity, cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against bacteriophage, viral infections. Other basic immune mechanisms evolved in ancien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PFOA
Perfluorooctanoic acid (PFOA; conjugate base perfluorooctanoate; also known colloquially as C8, from its chemical formula C8HF15O2) is a perfluorinated carboxylic acid produced and used worldwide as an industrial surfactant in chemical processes and as a chemical precursor. PFOA is considered a surfactant, or fluorosurfactant, due to its chemical structure, which consists of a perfluorinated, ''n''-heptyl "tail group" and a carboxylic acid "head group". The head group can be described as hydrophilic while the fluorocarbon tail is both hydrophobic and lipophobic. The International Agency for Research on Cancer (IARC) has classified PFOA as carcinogenic to humans. PFOA is one of many synthetic organofluorine compounds collectively known as per- and polyfluoroalkyl substances (PFASs). Many PFAS such as PFOS, PFOA are a concern because they do not break down via natural processes and are commonly described as persistent organic pollutants or "forever chemicals". They can also m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic bonding. The loosely defined term electropositivity is the opposite of electronegativity: it characterizes an element's tendency to donate valence electrons. On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more "pull" it will have on electrons) and the number and lo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon–fluorine Bond
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest single bonds in chemistry (after the B–F single bond, Si–F single bond, and H–F single bond), and relatively short, due to its partial ionic character. The bond also strengthens and shortens as more fluorines are added to the same carbon on a chemical compound. For this reason, fluoroalkanes like tetrafluoromethane (carbon tetrafluoride) are some of the most unreactive organic compounds. Electronegativity and bond strength The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine bond a significant polarity or dipole moment. The electron density is concentrated around the fluorine, leaving the carbon relatively electron poor. This introduces ionic character to the bond through partial charges (Cδ+—Fδ−). The partial charges on the fluorine and carbon ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipophobicity
Lipophobicity, also sometimes called lipophobia (from the Greek λιποφοβία from λίπος ''lipos'' "fat" and φόβος ''phobos'' "fear"), is a chemical property of chemical compounds which means " fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents. From the other point of view, they do not absorb fats. "Oleophobic" (from the Latin ''oleum'' "oil", Greek ελαιοφοβικό ''eleophobico'' from έλαιο ''eleo'' "oil" and φόβος ''phobos'' "fear") refers to the physical property of a molecule that is seemingly repelled from oil. (Strictly speaking, there is no repulsive force involved; it is an absence of attraction.) The most common lipophobic substance is water. Fluorocarbons are also lipophobic/oleophobic in addition to being hydrophobic. Uses A lipophobic coating has been used on the touchscreens of Apple's iPhones since the 3GS, their iPads, Nokia's N9 and Lumia devices, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface Tension
Surface tension is the tendency of liquid surfaces at rest to shrink into the minimum surface area possible. Surface tension (physics), tension is what allows objects with a higher density than water such as razor blades and insects (e.g. Gerridae, water striders) to float on a water surface without becoming even partly submerged. At liquid–air interfaces, surface tension results from the greater attraction of liquid molecules to each other (due to Cohesion (chemistry), cohesion) than to the molecules in the air (due to adhesion). There are two primary mechanisms in play. One is an inward force on the surface molecules causing the liquid to contract. Second is a tangential force parallel to the surface of the liquid. This ''tangential'' force is generally referred to as the surface tension. The net effect is the liquid behaves as if its surface were covered with a stretched elastic membrane. But this analogy must not be taken too far as the tension in an elastic membrane i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... Carboxylate esters have the general formula (also written as ), where R and R′ are organic groups. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. : Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organisation For Economic Co-operation And Development
The Organisation for Economic Co-operation and Development (OECD; , OCDE) is an international organization, intergovernmental organization with 38 member countries, founded in 1961 to stimulate economic progress and international trade, world trade. It is a forum (legal), forum whose member countries describe themselves as committed to democracy and the market economy, providing a platform to compare policy experiences, seek answers to common problems, identify good practices, and coordinate domestic and international policies of its members. The majority of OECD members are generally regarded as developed country, developed countries, with High-income economy, high-income economies, and a very high Human Development Index. their collective population is 1.38 billion people with an average life expectancy of 80 years and a median age of 40, against a global average of 30. , OECD Member countries collectively comprised 62.2% of list of countries by GDP (nominal), global nom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |