|
Perchloryl Fluoride
Perchloryl fluoride is a reactive gas with the chemical formula . It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid. In spite of its small enthalpy of formation (Δf''H''° = ), it is kinetically stable, decomposing only at 400 °C. It is quite reactive towards reducing agents and anions, however, with the chlorine atom acting as an electrophile. It reacts explosively with reducing agents such as metal amides, metals, hydrides, etc. Its hydrolysis in water occurs very slowly, unlike that of chloryl fluoride. Synthesis and chemistry Perchloryl fluoride is produced primarily by the fluorination of perchlorates. The initial syntheses in the early 1950s used fluorine gas or fluorides and anodic oxidation as the fluorinating agents, but these give explosive gaseous mixtures. A common fluorinator in modern syntheses is antimony pentafluoride: : Alt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rahway, New Jersey
Rahway () is a city (New Jersey), city in southern Union County, New Jersey, Union County, in the U.S. state of New Jersey. A bedroom community of New York City, it is centrally located in the Rahway River, Rahway Valley region, in the New York metropolitan area. The city is southwest of Manhattan and west of Staten Island. Built on the navigable Rahway River, it was an industrial and artisanal craft city for much of its history. The city has increasingly reinvented itself in recent years as a diverse regional hub for the arts and biotechnology, biological sciences, with a new global headquarters for Merck & Co. As of the 2020 United States census, the city's population was 29,556, its highest United States census, decennial count ever and an increase of 2,210 (+8.1%) from the 27,346 recorded at the 2010 United States census, 2010 census, which in turn reflected an increase of 846 (+3.2%) from the 26,500 counted in the 2000 United States census, 2000 census. History In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentafluoride
Antimony pentafluoride is the inorganic compound with the formula Sb F5. This colorless, viscous liquid is a strong Lewis acid and a component of the superacid fluoroantimonic acid, formed upon mixing liquid HF with liquid SbF5 in 1:1 ratio. It is notable for its strong Lewis acidity and the ability to react with almost all known compounds. Preparation Antimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim :SbCl5 + 5 HF → SbF5 + 5 HCl It can also be prepared from antimony trifluoride and fluorine. Structure and chemical reactions In the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture). The material adopts a more complicated structure in the liquid and solid states. The liquid contains polymers whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromine Pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subsequent analysis. It has also been tested as an oxidizer in liquid rocket propellants and is used as a fluorinating agent in the processing of uranium. Preparation BrF5 was first prepared in 1931 by the direct reaction of bromine and fluorine. This reaction is suitable for the preparation of large quantities, and is carried out at temperatures over with an excess of fluorine: :Br2 + 5 F2 → 2 BrF5 For the preparation of smaller amounts, potassium bromide is used: :KBr + 3 F2 → KF + BrF5 This route yields BrF5 almost completely free of trifluorides and other impurities. Reactions BrF5 reacts with water to form bromic acid and hydrofluoric acid: :BrF5 + 3 H2O → HBrO3 + 5 HF It is an extremely effective fluorinating agent, bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine Pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula . This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963. Preparation Some of the earliest research on the preparation was classified. It was first prepared by fluorination of chlorine trifluoride at high temperatures and high pressures: : : : : catalyzes this reaction. Certain metal fluorides, , e.g. (potassium tetrafluorochlorate(III)), (rubidium tetrafluorochlorate(III)), (caesium tetrafluorochlorate(III)), react with to produce and the corresponding alkali metal fluoride. Reactions In a highly exothermic reaction, reacts with water to produce chloryl fluoride and hydrogen fluoride: : It is also a strong fluorinating agent. At room temperature it reacts readily with all elements (including otherwise "inert" elem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Rocket Fuel
The highest specific impulse chemical rockets use liquid propellants (liquid-propellant rockets). They can consist of a single chemical (a monopropellant) or a mix of two chemicals, called bipropellants. Bipropellants can further be divided into two categories; hypergolic propellants, which ignite when the fuel and oxidizer make contact, and non-hypergolic propellants which require an ignition source. About 170 different propellants made of liquid fuel have been tested, excluding minor changes to a specific propellant such as propellant additives, corrosion inhibitors, or stabilizers. In the U.S. alone at least 25 different propellant combinations have been flown. Many factors go into choosing a propellant for a liquid-propellant rocket engine. The primary factors include ease of operation, cost, hazards/environment and performance. History Development in early 20th century Konstantin Tsiolkovsky proposed the use of liquid propellants in 1903, in his article ''Exploration of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
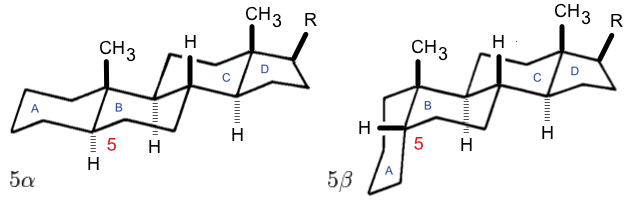
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The Aromatic sulfonation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is the addition of alkyl groups to molecules, often by alkylating agents such as Haloalkane, alkyl halides. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Bisulfate
Potassium bisulfate (potassium bisulphate) is an inorganic compound with the chemical formula KHSO4 and is the potassium acid salt of sulfuric acid. It is a white, water-soluble solid. Preparation More than 1 million tons were produced in 1985 as the initial stage in the Mannheim process for producing potassium sulfate. The relevant conversion is the exothermic reaction of potassium chloride and sulfuric acid: : Potassium bisulfate is a by-product in the production of nitric acid from potassium nitrate and sulfuric acid: : Chemical properties Thermal decomposition of potassium bisulfate forms potassium pyrosulfate: : Above 600 °C potassium pyrosulfate converts to potassium sulfate and sulfur trioxide: : Uses Potassium bisulfate is commonly used to prepare potassium bitartrate for winemaking. Potassium bisulfate is also used as a disintegrating agent in analytical chemistry or as a precursor to prepare potassium persulfate, a powerful oxidizing agent An oxidizing ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorosulfuric Acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substituting a fluorine atom for one of the hydroxyl groups. It is a colourless liquid, although commercial samples are often yellow.Erhardt Tabel, Eberhard Zirngiebl, Joachim Maas "Fluorosulfuric Acid" in "Ullmann's Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. Properties Fluorosulfuric acid is a free-flowing colorless liquid. It is soluble in polar organic solvents (e.g. nitrobenzene, acetic acid, and ethyl acetate), but poorly soluble in nonpolar solvents such as alkanes. is one of the strongest known simple Brønsted acids. It has an ''H''0 value of −15.1 compared to −12 for sulfuric acid. The combination of and the Lewis acid antimony pentafluoride produces "Magic acid", which is a far stronger protonating ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Perchlorate
Potassium perchlorate is the inorganic salt with the chemical formula K Cl O4. Like other perchlorates, this salt is a strong oxidizer when the solid is heated at high temperature, although it usually reacts very slowly in solution with reducing agents or organic substances. This colorless crystalline solid is a common oxidizer used in fireworks, ammunition percussion caps, and explosive primers, and is used variously in propellants, flash compositions, stars, and sparklers. It has been used as a solid rocket propellant, although in that application it has mostly been replaced by the more performant ammonium perchlorate. KClO4 has a relatively low solubility in water (1.5 g in 100 mL of water at 25 °C). Production Potassium perchlorate is prepared industrially by treating an aqueous solution of sodium perchlorate with potassium chloride. This single precipitation reaction exploits the low solubility of KClO4, which is about 1/100 as much as the solubili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |