|
Pentanenitrile
Pentanenitrile, valeronitrile or butyl cyanide is a nitrile with the formula C4H9CN. This can be written BuCN, with Bu representing an ''n''-butyl (linear butyl group). Production Pentanenitrile can be produced by heating 1-chlorobutane with sodium cyanide in dimethyl sulfoxide. This reaction takes about 20 minutes, keeping the temperature below 160 °C. The yield is about 93%. Another way to get the substance is by heating valeraldehyde with hydroxylamine. Pentanenitrile is contained in bone oil. Properties The pentanenitrile molecule is flexible and can adopt a number of different conformers, so that it will naturally be a mixture. These conformers are called anti-anti (30%), anti-gauche (46%), gauche-anti, gauche-gauche-cis, and gauche-gauche-trans. Biology Pentanenitrile is toxic to animals, and produces its action by the liberation of cyanide by cytochrome P450. The cyanide is detoxified and excreted in urine as thiocyanate. Pentanenitrile is found in ''Brassica'' s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Sulfoxide
Dimethyl sulfoxide (DMSO) is an organosulfur compound with the formula . This colorless liquid is the sulfoxide most widely used commercially. It is an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water. It has a relatively high boiling point. DMSO is metabolised to compounds that leave a garlic-like taste in the mouth after DMSO is absorbed by skin. In terms of chemical structure, the molecule has idealized Cs symmetry. It has a trigonal pyramidal molecular geometry consistent with other three-coordinate S(IV) compounds, with a nonbonded electron pair on the approximately tetrahedral sulfur atom. Synthesis and production Dimethyl sulfoxide was first synthesized in 1866 by the Russian scientist Alexander Zaytsev, who reported his findings in 1867. Its modern use as an industrial solvent began through popularization by Thor Smedslund at the Stepan Chemical Company. Dimeth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
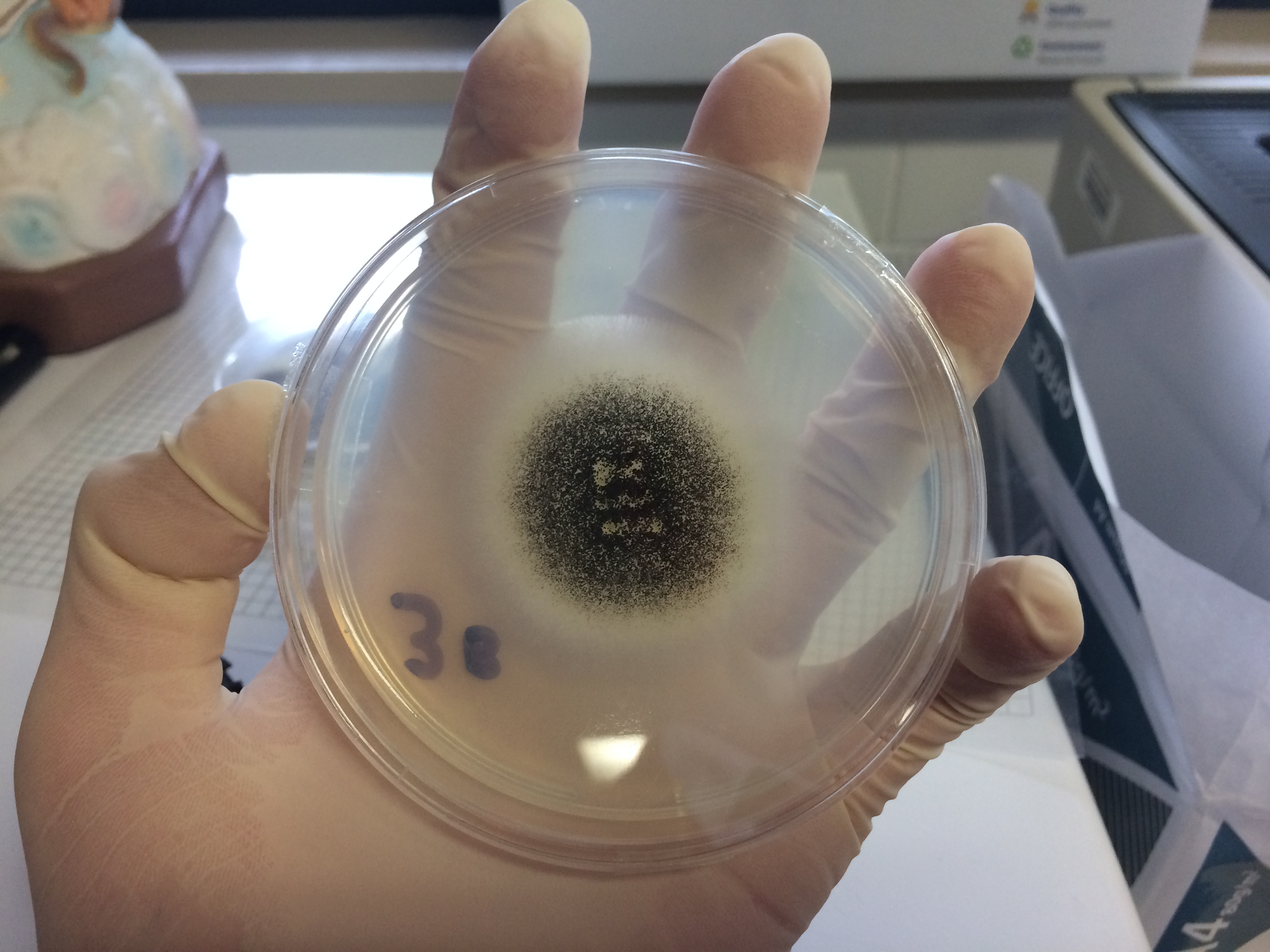
Aspergillus Niger
''Aspergillus niger'' is a mold classified within the ''Nigri'' section of the ''Aspergillus'' genus. The ''Aspergillus'' genus consists of common molds found throughout the environment within soil and water, on vegetation, in fecal matter, on decomposing matter, and suspended in the air. Species within this genus often grow quickly and can sporulate within a few days of germination. A combination of characteristics unique to ''A. niger'' makes the microbe invaluable to the production of many acids, proteins and bioactive compounds. Characteristics including extensive metabolic diversity, high production yield, secretion capability, and the ability to conduct post-translational modifications are responsible for ''A. niger's'' robust production of secondary metabolites. ''A. niger's'' capability to withstand extremely acidic conditions makes it especially important to the industrial production of citric acid. ''A. niger'' causes a disease known as "black mold" on certain fruits and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusarium Oxysporum
''Fusarium oxysporum'' (Schlecht as emended by Snyder and Hansen), an ascomycete fungus, comprises all the species, varieties and forms recognized by Wollenweber and Reinking within an infrageneric grouping called section Elegans. It is part of the family Nectriaceae. Although their predominant role in native soils may be as harmless or even beneficial plant endophytes or soil saprophytes, many strains within the ''F. oxysporum'' complex are soil borne pathogens of plants, especially in agricultural settings. Taxonomy While the species, as defined by Snyder and Hansen, has been widely accepted for more than 50 years, more recent work indicates this taxon is actually a genetically heterogeneous polytypic morphospecies, whose strains represent some of the most abundant and widespread microbes of the global soil microflora. Genome The ' family of transposable elements was first discovered by Daboussi ''et al.'', 1992 in several ''formae speciales'' and Davière ''et al.'', 2001 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibberella Intermedia
''Gibberella'' is a genus of fungi in the family Nectriaceae. In 1926, Japanese scientists observed that rice plants infected with ''Gibberella'' had abnormally long stems (" foolish seedling disease"). A substance, gibberellin, was derived from this fungus. Gibberellin is a plant hormone that promotes cell elongation, flower formation, and seedling growth. Etymology Pier Andrea Saccardo named the genus "Gibberella" because of the hump (Latin, ''gibbera'') on the fungal perithecium.''GIBBERELLA'' FROM ''A'' (VENACEAE) TO ''Z' (EAE) , by Anne E. Desjardins; originally published in '''', 2003. 41:177–98; [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valeric Acid
Valeric acid or pentanoic acid is a straight-chain alkyl carboxylic acid with the chemical formula . Like other low-molecular-weight carboxylic acids, it has an unpleasant odor. It is found in the perennial flowering plant '' Valeriana officinalis'', from which it gets its name. Its primary use is in the synthesis of its esters. Salts and esters of valeric acid are known as valerates or pentanoates. Volatile esters of valeric acid tend to have pleasant odors and are used in perfumes and cosmetics. Several, including ethyl valerate and pentyl valerate are used as food additives because of their fruity flavors. History Valeric acid is a minor constituent of the perennial flowering plant valerian (''Valeriana officinalis''), from which it gets its name. The dried root of this plant has been used medicinally since antiquity. The related isovaleric acid shares its unpleasant odor and their chemical identity was investigated by oxidation of the components of fusel alcohol, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brassica
''Brassica'' () is a genus of plants in the cabbage and mustard family (Brassicaceae). The members of the genus are informally known as cruciferous vegetables, cabbages, mustard plants, or simply brassicas. Crops from this genus are sometimes called ''cole crops''derived from the Latin ''caulis'', denoting the stem or stalk of a plant. The genus ''Brassica'' is known for its important agricultural and horticultural crops and also includes a number of weeds, both of wild taxa and escapees from cultivation. ''Brassica'' species and varieties commonly used for food include bok choy, broccoli, cauliflower, cabbage, choy sum, kohlrabi, napa cabbage, rutabaga, turnip and some seeds used in the production of canola oil and the condiment mustard. Over 30 wild species and hybrids are in cultivation, plus numerous cultivars and hybrids of cultivated origin. Most are seasonal plants ( annuals or biennials), but some are small shrubs. ''Brassica'' plants have been the subject o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanate
Thiocyanates are salts containing the thiocyanate anion (also known as rhodanide or rhodanate). is the conjugate base of thiocyanic acid. Common salts include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics. Thiocyanate is analogous to the cyanate ion, , wherein oxygen is replaced by sulfur. is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide: : : The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body. Oxidation of thiocyanate inevitably produces hydrogen sulfate. The other product depe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for example, they have not been found in ''Escherichia coli''. In mammals, these enzymes oxidize steroids, fatty acids, xenobiotics, and participate in many biosyntheses. By hydroxylation, CYP450 enzymes convert xenobiotics into hydrophilic derivatives, which are more readily excreted. P450s are, in general, the terminal oxidase enzymes in electron transfer chains, broadly categorized as P450-containing systems. The term "P450" is derived from the spectrophotometry, spectrophotometric peak at the wavelength of the absorption spectroscopy, absorption maximum of the enzyme (450 nanometre, nm) when it is in the redox, reduced state and complexed with carbon monoxide. Most P450s require a protein partner to deliver one or more electrons to reduc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dippel's Oil
Dippel's oil (sometimes referred to as bone oil) is a nitrogenous by-product of the destructive distillation of bones. A dark, viscous, tar-like liquid with an unpleasant smell, it is named after its inventor, Johann Konrad Dippel. The oil consists of aliphatic chains, with nitrogen functional groups including pyrroles, pyridines and nitriles, as well as other nitrogenous compounds. Dippel's oil had a number of uses, which are mostly obsolete. Its primary use was as an animal and insect repellent. It saw limited use as a chemical warfare harassing agent during the desert campaign of World War II. The oil was used to render wells undrinkable and thus deny their use to the enemy. By not being lethal, the oil was claimed to not be in breach of the Geneva Protocol. See also * Neatsfoot oil, another bone-derived oil * Bone char Bone char () is a porous, black, granular material produced by charring animal bones. Its composition varies depending on how it is made; however, it con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution or more often as one of its salts such as hydroxylammonium sulfate, a water-soluble solid. Hydroxylamine and its salts are consumed almost exclusively to produce Nylon-6. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex (zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentanal
Pentanal (also called valeraldehyde) is the organic compound with molecular formula . Classified as an alkyl aldehyde, it is a colorless volatile liquid. Its odor is described as fermented, bready, fruity, nutty, berry. Production Pentanal is obtained by hydroformylation of butene. Also C4 mixtures can be used as starting material like the so-called raffinate II, which is produced by steam cracking and contains (''Z'')- and (''E'')-2-butene, 1-butene, butane and isobutane. The conversion to the product is accomplished with synthesis gas in the presence of a catalyst consisting of a rhodium-bisphosphite complex and a sterically hindered secondary amine with a selectivity toward pentanal of at least 90%. Use Pentanal undergoes the reactions characteristic of any alkyl aldehyde, i.e., oxidations, condensations, and reductions. 2-Octanone, produced for use in the fragrance industry, is obtained by the condensation of acetone and pentanal, followed by hydrogenation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |