|
Pentaerythritol Tetrakis(3-mercaptopropionate)
Pentaerythritol tetrakis(3-mercaptopropionate) is an organic compound which is derived from pentaerythritol fully esterified with four equivalents of 3-mercaptopropionic acid. It is a colorless liquid at room temperature. Uses Pentaerythritol tetrakis(3-mercaptopropionate) is a common thiol monomer reacted with alkenes in the thiol-ene reaction to form polymeric networks. Being functionalized with four thiol groups, it can react with multifunctional alkenes to form thiol-ene networks. References Monomers Propionate esters Thiols {{ester-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
PubChem
PubChem is a database of Chemistry, chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1,000 bonds. More than 80 database vendors contribute to the growing PubChem database. History PubChem was released in 2004 as a component of the Molecular Libraries Program (MLP) of the NIH. As of November 2015, PubChem contains more than 150 million depositor-provided substance descriptions, 60 million unique chemical structures, and 225 million biological activity test results (from over 1 million assay experiments performe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
ChemSpider
ChemSpider is a freely accessible online chemical database, database of chemicals owned by the Royal Society of Chemistry. It contains information on more than 100 million molecules from over 270 data sources, each of them receiving a unique identifier called ChemSpider Identifier. Sources The database sources include: Professional databases * EPA DSSTox * Food and Drug Administration (United States), U.S. Food and Drug Administration (FDA) * Human Metabolome Database * Journal of Heterocyclic Chemistry * KEGG * KUMGM * LeadScope * LIPID MAPS, LipidMAPS * Marinlit * MDPI * MICAD * MLSMR * MMDB * MOLI * MTDP * Nanogen * Nature Chemical Biology * NCGC * NIAID * National Institutes of Health (NIH) * NINDS Approved Drug Screening Program * NIST * NIST Chemistry WebBook * NMMLSC * NMRShiftDB * PANACHE * PCMD * PDSP * Peptides (journal), Peptides * Prous Science Drugs of the Future * QSAR * R&D Chemicals * San Diego Center for Chemical Genomics * SGCOxCompounds, SGCStoCompounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pentaerythritol
Pentaerythritol is an organic compound with the formula C(CH2OH)4. The molecular structure can be described as a neopentane with one hydrogen atom in each methyl group replaced by a hydroxyl (–OH) group. It is therefore a polyol, specifically a tetrol. Pentaerythritol is a white solid. It is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products. The word pentaerythritol is a blend of ''penta-'' in reference to its five carbon atoms and ''erythritol'', which also possesses 4 alcohol groups. Synthesis Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand. It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde to give pentaerythrose (CAS: 3818-32-4), followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product plus formate ion. : Uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well (e.g. amides), but not according to the IUPAC. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Lactones are cyclic carboxylic esters; naturally occurring lactones are mainly 5- and 6-membered ring lactones. Lactones contribute to the aroma of fruits, butter, cheese, vegetables like celery and other foods. Esters can be formed from oxoacids (e.g. esters of acetic acid, carbonic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
3-mercaptopropionic Acid
3-Mercaptopropionic acid (3-MPA) is an organosulfur compound with the formula HSCH2CH2CO2H. It is a bifunctional molecule, containing both carboxylic acid and thiol groups. It is a colorless oil. It is derived from the addition of hydrogen sulfide to acrylic acid. Reactions and uses It is competitive inhibitor of glutamate decarboxylase, and therefore acts as a convulsant. It has higher potency and faster onset of action compared to allylglycine. It is used to prepare hydrophilic gold nanoparticles, exploiting the affinity of gold for sulfur ligands. It is esterified with polyols to form thiol-based polymer cross-linking agents such as pentaerythritol-based pentaerythritol tetrakis(3-mercaptopropionate). See also * Allylglycine * Thiolactic acid Thiolactic acid is the organosulfur compound with the formula . The molecule contains both carboxyl and thiol functional groups, and respectively. It is structurally related to lactic acid by the interchange of for . It is a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thiol-ene Reaction
In organosulfur chemistry, the thiol-ene reaction (also alkene hydrothiolation) is an organic reaction between a thiol () and an alkene () to form a thioether (). This reaction was first reported in 1905, but it gained prominence in the late 1990s and early 2000s for its feasibility and wide range of applications. This reaction is accepted as a click chemistry reaction given the reactions' high yield, stereoselectivity, high rate, and thermodynamic driving force. The reaction results in an anti-Markovnikov addition of a thiol compound to an alkene. Given the stereoselectivity, high rate and yields, this synthetically useful reaction may underpin future applications in material and biomedical sciences. Mechanisms Radical addition Thiol-ene additions are known to proceed through two mechanisms: free-radical additions and catalyzed Michael additions. Free-radical additions can be initiated by light, heat or radical initiators, which form a thiyl radical species. The radical t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Angewandte Chemie International Edition
''Angewandte Chemie'' (, meaning "Applied Chemistry") is a weekly peer-reviewed scientific journal that is published by Wiley-VCH on behalf of the German Chemical Society (Gesellschaft Deutscher Chemiker). Publishing formats include feature-length reviews, short highlights, research communications, minireviews, essays, book reviews, meeting reviews, correspondences, corrections, and obituaries. This journal contains review articles covering all aspects of chemistry. According to the ''Journal Citation Reports'', the journal had a 2023 impact factor of 16.1. Editions The journal appears in two editions with separate volume and page numbering: a German edition, ''Angewandte Chemie'', and a fully English-language edition, ''Angewandte Chemie International Edition''. The editions are identical in content with the exception of occasional reviews of German-language books or German translations of IUPAC recommendations. Publication history In 1887, Ferdinand Fischer established the '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Monomers
A monomer ( ; ''wikt:mono-, mono-'', "one" + ''wikt:-mer, -mer'', "part") is a molecule that can chemical reaction, react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a polymer#Monomers and repeat units, homopolymer. Many polymers are copolymers, meaning that they are derived from two diff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
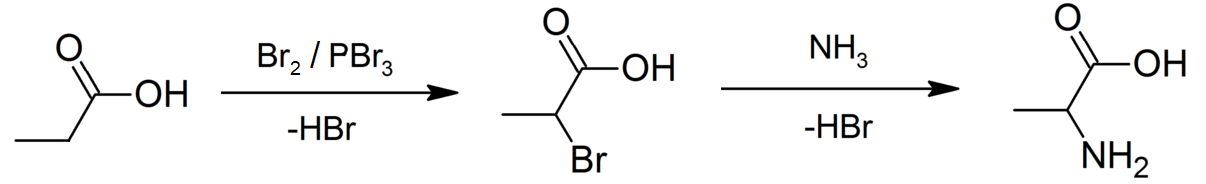
Propionate Esters
Propionic acid (, from the Greek language, Greek words πρῶτος : ''prōtos'', meaning "first", and πίων : ''píōn'', meaning "fat"; also known as propanoic acid) is a naturally occurring carboxylic acid with chemical formula . It is a liquid with a pungent and unpleasant smell somewhat resembling body odor. The anion as well as the Carboxylate salt, salts and esters of propionic acid are known as propionates or propanoates. About half of the world production of propionic acid is consumed as a preservative for both animal feed and food for human consumption. It is also useful as an intermediate in the production of other chemicals, especially polymers. History Propionic acid was first described in 1844 by Johann Gottlieb, who found it among the degradation products of sugar. Over the next few years, other chemists produced propionic acid by different means, none of them realizing they were producing the same substance. In 1847, French chemist Jean-Baptiste Dumas esta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |