|
Pegargiminase
Pegargiminase (also known as pegylated arginine deiminase) is an investigational drug used in arginine deprivation therapy for treating cancers deficient in argininosuccinate synthetase 1 (ASS1). It is a recombinant form of the enzyme arginine deiminase cloned from the bacteria '' Mycoplasma hominus'' and synthesized in ''Escherichia coli''. It has been pegylated to improve the half-life and reduce immunogenicity Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injecti .... References {{Reflist Recombinant proteins Orphan drugs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argininosuccinate Synthetase 1
Argininosuccinate synthetase is an enzyme that in humans is encoded by the ''ASS1'' gene. The protein encoded by this gene catalyzes the penultimate step of the arginine biosynthetic pathway. There are approximately 10 to 14 copies of this gene including the pseudogenes scattered across the human genome, among which the one located on chromosome 9 appears to be the only functional gene for argininosuccinate synthetase. Two transcript variants encoding the same protein have been found for this gene. Clinical significance Mutations in the chromosome 9 copy of ASS cause citrullinemia. Arginine is considered a non-essential amino acid since normal cells can synthesize it from citrulline and aspartate Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of protein ... using the enzymes argininosucci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Investigational Drug
The United States Food and Drug Administration's Investigational New Drug (IND) program is the means by which a pharmaceutical company obtains permission to start human clinical trials and to ship an experimental drug across state lines (usually to clinical investigators) before a marketing application for the drug has been approved. Regulations are primarily at . Similar procedures are followed in the European Union, Japan, and Canada due to regulatory harmonization efforts by the International Council for Harmonisation. Types * Research or investigator INDs are non-commercial INDs filed by researchers to study an unapproved drug or to study an approved drug for a new indication or in a new patient population. * Emergency Use INDs, also called compassionate use or single-patient INDs, are filed for emergency use of an unapproved drug when the clinical situation does not allow sufficient time to submit an IND in accordance wit21 CFR §§ 312.23 312.24. These are most commonly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine Deiminase
In enzymology, an arginine deiminase () is an enzyme that catalyzes the chemical reaction :L-arginine + H2O \rightleftharpoons L-citrulline + NH3 Thus, the two substrates of this enzyme are L-arginine and H2O, whereas its two products are L-citrulline and NH3. This enzyme belongs to the family of hydrolases, those acting on carbon-nitrogen bonds other than peptide bonds, specifically in linear amidines. The systematic name of this enzyme class is L-arginine iminohydrolase. Other names in common use include arginine dihydrolase, citrulline iminase, and L-arginine deiminase. This enzyme participates in arginine and proline metabolism. This enzyme is widely expressed in bacteria, including streptococcus and actinomyces. The bacterial arginine deiminase expression could be regulated by various environmental factors. Recently, a new enzyme that catalyzes the chemical reaction :L-arginine + 2H2O \rightleftharpoons L-ornithine + 2NH3 + CO2 was identified in cyanobacteria which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mycoplasma
''Mycoplasma'' is a genus of bacteria that, like the other members of the class ''Mollicutes'', lack a cell wall, and its peptidoglycan, around their cell membrane. The absence of peptidoglycan makes them naturally resistant to antibiotics such as the beta-lactam antibiotics that target cell wall synthesis. They can be parasitic or saprotrophic. In casual speech, the name ''"mycoplasma"'' (plural ''mycoplasmas'' or ''mycoplasms'') generally refers to all members of the class Mollicutes. In formal scientific classification, the designation ''Mycoplasma'' refers exclusively to the genus, a member of the Mycoplasmataceae, the only family in the order Mycoplasmatales (see "scientific classification"). In 2018, ''Mycoplasma'' was split with many clinically significant species moved to other genera in Mollicutes; see the page Mollicutes for an overview. Etymology The term "mycoplasma", from the Greek μύκης, ' (fungus) and πλάσμα, ' (formed), was first used by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are part of the normal microbiota of the gut, where they constitute about 0.1%, along with other facultative anaerobes. These bacteria are mostly harmless or even beneficial to humans. For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by harmful pathogenic bacteria. These mutually beneficial relationships between ''E. coli'' and humans are a type of mutualistic biological relationship—where both the humans and the ''E. coli'' are benefitting each other. ''E. coli'' is expelled into the environment within fecal matter. The bacterium grows massi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pegylated
PEGylation (or pegylation) is the process of both covalent and non-covalent attachment or amalgamation of polyethylene glycol (PEG, in pharmacy called macrogol) polymer chains to molecules and macrostructures, such as a drug, therapeutic protein or vesicle, which is then described as PEGylated. PEGylation affects the resulting derivatives or aggregates interactions, which typically slows down their coalescence and degradation as well as elimination in vivo. PEGylation is routinely achieved by the incubation of a reactive derivative of PEG with the target molecule. The covalent attachment of PEG to a drug or therapeutic protein can "mask" the agent from the host's immune system (reducing immunogenicity and antigenicity), and increase its hydrodynamic size (size in solution), which prolongs its circulatory time by reducing renal clearance. PEGylation can also provide water solubility to hydrophobic drugs and proteins. Having proven its pharmacological advantages and acceptability, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunogenicity
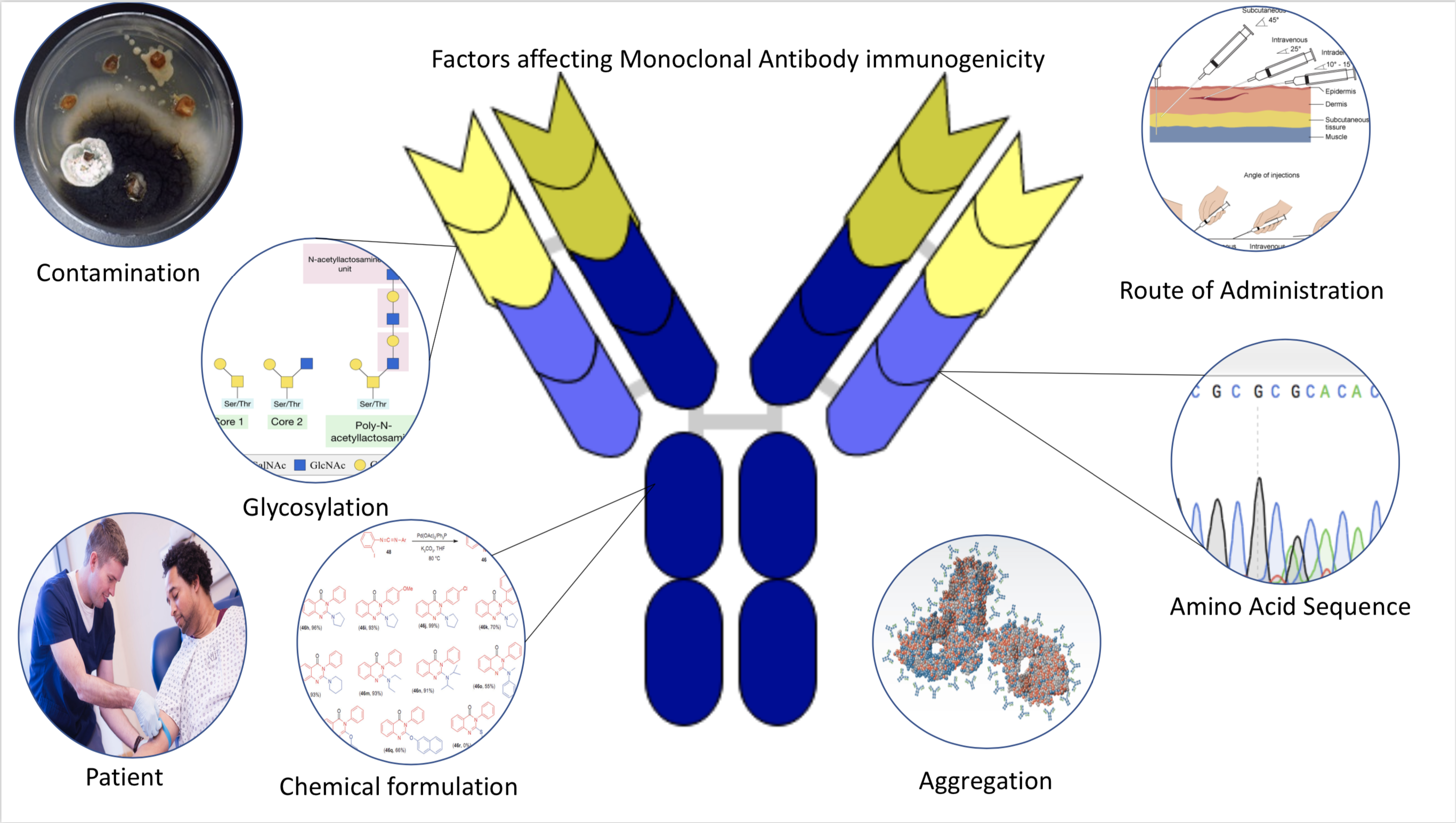
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant Proteins
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining two or more fragments from different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, differing only in the nucleotide sequence. Recombinant DNA molecules are sometimes called chimeric DNA because they can be made of material from two different species like the mythical chimera. rDNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA can be joined to bacterial DNA, or human DNA can be joined with fungal D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |