|
Pawhuskin B
Pawhuskin A is a naturally occurring prenylated stilbene isolated from ''Dalea purpurea'' which acts as a competitive silent antagonist of the κ-, μ-, and δ-opioid receptors (Ke = 203 nM, 570 nM, and 2900 nM, respectively). The compound was named after Pawhuska, Oklahoma, a place near where the samples of ''Dalea purpurea'' that led to its discovery were taken from. Other isolates of the plant with affinity for opioid receptors include Pawhuskin B and Pawhuskin C, though these compounds produce comparatively weak opioid receptor displacement (4.2–11.4 μM) relative to Pawhuskin A. ''Dalea purpurea'' was used in traditional Native American medicine to treat various ailments, and pawhuskin A and related isolates may be some of the constituents of the plant which underlay this use. See also * Amentoflavone * Catechin * Hyperoside * Salvinorin A Salvinorin A is the main active psychotropic molecule in '' Salvia divinorum''. Salvinorin A is considered an atypical d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical synthesis (both semisynthesis and total synthesis and have played a central role in the development of the field of organic chemistry by providing challenging synthetic targets). The term ''natural product'' has also been extended for commercial purposes to refer to cosmetics, dietary supplements, and foods produced from natural sources without added artificial ingredients. Within the field of organic chemistry, the definition of natural products is usually restricted to organic compounds isolated from natural sources that are produced by the pathways of primary or secondary metabolism. Within the field of medicinal chemistry, the definition is often further restricted to secondary metabolites. Secondary metabolites (or specialized meta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
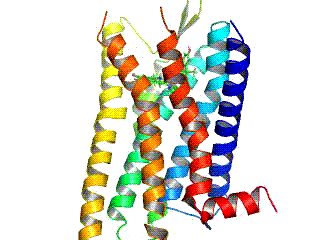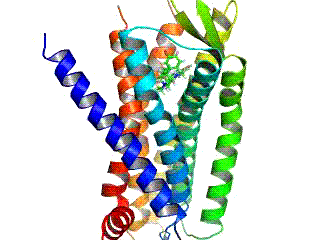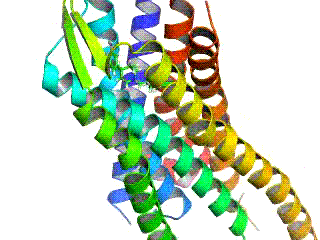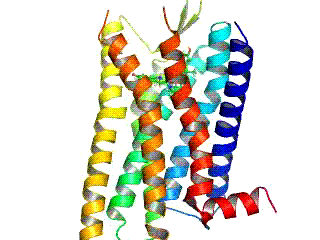
Opioid Receptor
Opioid receptors are a group of inhibitory G protein-coupled receptors with opioids as ligands. The endogenous opioids are dynorphins, enkephalins, endorphins, endomorphins and nociceptin. The opioid receptors are ~40% identical to somatostatin receptors (SSTRs). Opioid receptors are distributed widely in the brain, in the spinal cord, on peripheral neurons, and digestive tract. Discovery By the mid-1960s, it had become apparent from pharmacologic studies that opioids were likely to exert their actions at specific receptor sites, and that there were likely to be multiple such sites. Early studies had indicated that opiates appeared to accumulate in the brain. The receptors were first identified as specific molecules through the use of binding studies, in which opiates that had been labeled with radioisotopes were found to bind to brain membrane homogenates. The first such study was published in 1971, using 3H- levorphanol. In 1973, Candace Pert and Solomon H. Snyder publi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kappa-opioid Receptor Antagonists
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors. Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This effectively blocks the receptor, preventing the body from responding to opioids and endorphins. Some opioid antagonists are not pure antagonists but do produce some weak opioid partial agonist effects, and can produce analgesic effects when administered in high doses to opioid-naive individuals. Examples of such compounds include nalorphine and levallorphan. However, the analgesic effects from these specific drugs are limited and tend to be accompanied by dysphoria, most likely due to additional agonist action at the κ-opioid receptor. As they induce opioid withdrawal effects in people who are taking, or have recently used, opioid full agonists, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salvinorin A
Salvinorin A is the main active psychotropic molecule in '' Salvia divinorum''. Salvinorin A is considered an atypical dissociative hallucinogen. It is structurally distinct from other naturally occurring hallucinogens (such as DMT, psilocybin, and mescaline) because it contains no nitrogen atoms; hence, it is not an alkaloid (and cannot be rendered as a salt), but rather is a terpenoid. It also differs in subjective experience, compared to other hallucinogens, and has been described as having strong dissociative effects. Salvinorin A can produce psychoactive experiences in humans with a typical duration of action being several minutes to an hour or so, depending on the method of ingestion. Salvinorin A is found with several other structurally related salvinorins. Salvinorin is a ''trans''- neoclerodane diterpenoid. It acts as a kappa opioid receptor agonist and is the first known compound acting on this receptor that is not an alkaloid. History Salvinorin A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperoside
Hyperoside is a chemical compound. It is the 3-''O''-galactoside of quercetin. Natural occurrences Hyperoside has been isolated from ''Drosera rotundifolia'', from the Lamiaceae ''Stachys sp.'' and ''Prunella vulgaris'', from ''Rumex acetosella'', '' Cuscuta chinensis'' seeds, from St John's wort and from ''Camptotheca acuminata''. It is one of the phenolic compounds in the invasive plant ''Carpobrotus edulis'' and contributes to the antibacterial properties of the plant. In '' Rheum nobile'' and '' R. rhaponticum'', it serves as a UV blocker found in the bracts. It is also found in ''Geranium niveum ''Geranium niveum'' is a plant species in the genus ''Geranium''. It is a medicinal herb widely used by the Tarahumara Indians of Mexico. Geranin A Geranin A is an A type proanthocyanidin of the propelargonidin sub type. Its structure is ep ...'' and '' Taxillus kaempferi''.The constituents of Taxillus kaempferi and the host, Pinus thunbergii. I. Catechins and flavones of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catechin
Catechin is a flavan-3-ol, a type of secondary metabolite providing antioxidant roles in plants. It belongs to the subgroup of polyphenols called flavonoids. The name of the catechin chemical family derives from ''catechu'', which is the tannic juice or boiled extract of ''Mimosa catechu'' (''Acacia catechu'' L.f.). Chemistry Catechin possesses two benzene rings (called the A and B rings) and a dihydropyran heterocycle (the C ring) with a hydroxyl group on carbon 3. The A ring is similar to a resorcinol moiety while the B ring is similar to a catechol moiety. There are two chirality (chemistry), chiral centers on the molecule on carbons 2 and 3. Therefore, it has four diastereoisomers. Two of the isomers are in trans configuration, ''trans'' configuration and are called ''catechin'' and the other two are in cis configuration, ''cis'' configuration and are called ''epicatechin''. The most common catechin isomer is (+)-catechin. The other stereoisomer is (−)-catechin or ''en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amentoflavone
Amentoflavone is a biflavonoid (''bis''-apigenin coupled at 8 and 3 positions, or 3,8-biapigenin) constituent of a number of plants including ''Ginkgo biloba'', ''Chamaecyparis obtusa'' (hinoki), ''Biophytum sensitivum'', '' Selaginella tamariscina'', ''Hypericum perforatum'' (St. John's Wort) and '' Xerophyta plicata''. Amentoflavone can interact with many medications by being a potent inhibitor of CYP3A4 and CYP2C9, which are enzymes responsible for the metabolism of some drugs in the body. It is also an inhibitor of human cathepsin B. Amentoflavone has a variety of ''in vitro'' activities including antimalarial activity, anticancer activity (which may, at least in part, be mediated by its inhibition of fatty acid synthase), and antagonist activity at the κ-opioid receptor (''K''e = 490 nmol L−1) as well as activity at the allosteric benzodiazepine site of the GABAA receptor as a negative allosteric modulator. See also * Apigenin Apigenin (4� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Native American Ethnobotany
Indigenous peoples of North America used various plants for different purposes. For lists pertaining specifically to the Cherokee, Iroquois, Navajo, and Zuni, see Cherokee ethnobotany, Iroquois ethnobotany, Navajo ethnobotany, and Zuni ethnobotany. A *'' Abronia fragrans'' (snowball-sand verbena) Used as both food and medicine. See article for complete list of uses. * ''Acer glabrum'' var. ''douglasii'' (Douglas maple), used by Plateau tribes as a treatment for diarrhea. * ''Acer glabrum'' var. ''glabrum'' The Blackfoot take an infusion of the bark in the morning as a cathartic. The Okanagan-Colville, when hunting, use a branch tied in a knot and placed over the bear's tracks while hunting to stop the wounded bear. The Thompson people use a decoction of wood and bark taken for nausea caused by smelling a corpse. *''Acer negundo'' (box elder), used as food, lumber, and medicine. Please see article for full information. *''Acer saccharinum'' (silver maple), an infusion of bar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pawhuskin C
Pawhuskin A is a naturally occurring prenylated stilbene isolated from ''Dalea purpurea'' which acts as a competitive silent antagonist of the κ-, μ-, and δ-opioid receptors (Ke = 203 nM, 570 nM, and 2900 nM, respectively). The compound was named after Pawhuska, Oklahoma, a place near where the samples of ''Dalea purpurea'' that led to its discovery were taken from. Other isolates of the plant with affinity for opioid receptors include Pawhuskin B and Pawhuskin C, though these compounds produce comparatively weak opioid receptor displacement (4.2–11.4 μM) relative to Pawhuskin A. ''Dalea purpurea'' was used in traditional Native American medicine to treat various ailments, and pawhuskin A and related isolates may be some of the constituents of the plant which underlay this use. See also * Amentoflavone * Catechin * Hyperoside * Salvinorin A Salvinorin A is the main active psychotropic molecule in '' Salvia divinorum''. Salvinorin A is considered an atypical dis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |