|
Ozenoxacin
Ozenoxacin, sold under the brand names Ozanex and Xepi, is a quinolone antibiotic used for the treatment of impetigo. A 1% topical cream is approved for treatment of impetigo in Canada and in the United States. Ozenoxacin is active against some bacteria that have developed resistance to fluoroquinolone antibiotics. Mechanism of action Like other quinolone antibiotics, ozenoxacin targets DNA gyrase and topoisomerase IV. Its activity against bacteria with fluoroquinolone resistance is attributed to its evasion of bacterial efflux pumps. Chemistry Synthesis Ozenoxacin is synthesized by the Pd-catalyzed cross-coupling of a bromoquinolone and a pyridyl tributylstannane (Stille coupling). : The pyridyl tributylstannane is synthesized from the corresponding dihalopyridine. This is achieved through a sequence of nucleophilic aromatic substitution with methylamine, which is protected as the acetamide using acetic anhydride and this is converted to the organostannane through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topical Medication
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surface area, body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including Cream (pharmaceutical), creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be insufflation (medicine), inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a Human tooth, tooth. The word ''topical'' derives from Ancient Greek, Greek wikt:τοπικός, τοπικός ''topikos'', "of a place". Justification Topical drug delivery is a route of administering drugs via the Human skin, skin to provide topical therapeutic effects. As sk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylamine
Methylamine, also known as methanamine, is an organic compound with a formula of . This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine. Methylamine is sold as a solution in methanol, ethanol, tetrahydrofuran, or water, or as the anhydrous gas in pressurized metal containers. Industrially, methylamine is transported in its anhydrous form in pressurized railcars and tank trailers. It has a strong odor similar to rotten fish. Methylamine is used as a building block for the synthesis of numerous other commercially available compounds. Industrial production Methylamine has been produced industrially since the 1920s (originally by Commercial Solvents Corporation for dehairing of animal skins). This was made possible by and his wife Eugenia who discovered amination of alcohols, including methanol, on alumina or kaolin catalyst after WWI, filed two patent applications in 1919 and published an a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropyl Compounds
A cyclopropyl group is a chemical structure derived from cyclopropane; it is typically produced in a cyclopropanation reaction. The group has an empirical formula of C3H5 and chemical bonds from each of the three carbons to both of the other two. Structure and bonding Due to the unfavoured bond angles (60°), cyclopropyl groups are highly strained. Two orbital models were proposed to describe the bonding situation. The Coulson-Moffit model uses bent bonds. The C-C bonds are formed by overlap of two sp-hybrid orbitals. To adapt to the small bond angle, there is some rehybridization resulting in sp~5-hybrids for the ring bonds and sp~2 for the C-H bonds. This model resembles the banana bond model for C=C double bonds (τ bonds). Alternatively the structure can be explained with the Walsh model. Here the two sp-hybrids forming the ring bond are separated into one sp2-hybrid and one pure p-orbital. This corresponds to the π bond description of C=C double bonds. Cyclopropyl grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinolone Antibiotics
Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production. Quinolone antibiotics are classified into four generations based on their spectrum of activity and chemical modifications. The first-generation quinolones, such as nalidixic acid, primarily target Gram-negative bacteria and are mainly used for urinary tract infections. Second-generation quinolones introduced fluorine atoms into their structure, creating fluoroquinolones, which significantly expanded their antibacterial activity to include some Gram-positive bacteria. Third-generation fluoroquinolones further improved Gram-positive coverage, while fourth-generation fluoroquinolones offer broad-spectrum activity, including anaerobic bacteria. Only quinolone antibiotics in generation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
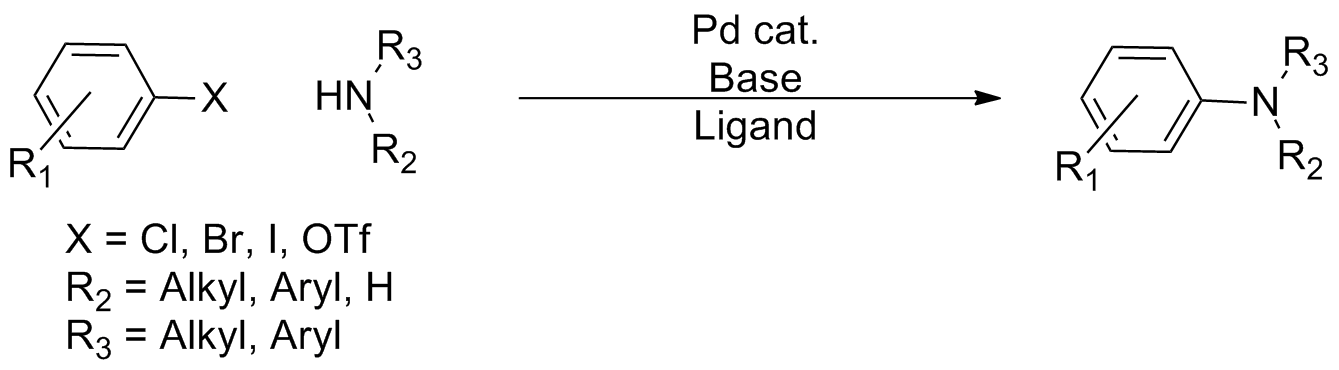
Buchwald–Hartwig Amination
In organic chemistry, the Buchwald–Hartwig amination is a chemical reaction for the synthesis of carbon–nitrogen bonds via the cross-coupling reaction, palladium-catalyzed coupling reactions of amines with aryl halides. Although Pd-catalyzed C–N couplings were reported as early as 1983, Stephen L. Buchwald and John F. Hartwig have been credited, whose publications between 1994 and the late 2000s established the scope of the transformation. The reaction's synthetic utility stems primarily from the shortcomings of typical methods (nucleophilic substitution, reductive amination, etc.) for the synthesis of aromatic bonds, with most methods suffering from limited substrate scope and functional group tolerance. The development of the Buchwald–Hartwig reaction allowed for the facile synthesis of aryl amines, replacing to an extent harsher methods (the Goldberg reaction, nucleophilic aromatic substitution, etc.) while significantly expanding the repertoire of possible bond format ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friedel–Crafts Reaction
The Friedel–Crafts reactions are a set of organic reaction, reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an Aromatic hydrocarbon, aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution. Alkylation With alkenes In commercial applications, the alkylating agents are generally alkenes, some of the largest scale reactions practiced in industry. Such alkylations are of major industrial importance, e.g. for the production of ethylbenzene, the precursor to polystyrene, from benzene and ethylene and for the production of cumene from benzene and propene in cumene process: : : Industrial production typically uses solid acids derived from a zeolite as the catalyst. With alkyl halides Friedel–Crafts alkylation involves the alkylation of an aromatic ring. Traditionally, the alkylating agents are alkyl halides. Many alkylating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition Reaction
In organic chemistry, the Michael reaction or Michael 1,4 addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon–carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tributyltin
Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the group, with a prominent example being tributyltin oxide. For 40 years TBT was used as a biocide in anti-fouling paint, commonly known as bottom paint, applied to the hulls of oceangoing vessels. Bottom paint improves ship performance and durability as it reduces the rate of biofouling, the growth of organisms on the ship's hull. The TBT slowly leaches out into the marine environment where it is highly toxic toward nontarget organisms. TBT toxicity can lead to biomagnification or bioaccumulation within such nontarget organisms like invertebrates, vertebrates, and a variety of mammals. TBT is also an obesogen. After it led to collapse of local populations of organisms, TBT was banned. Chemical properties TBT, or tributyltin, tributylstannyl or tributyl stannic hydride compounds are organotin compounds. They have three butyl groups covalently bonded to a tin(IV) atom.Davies, Alwyn Georg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of cellulose acetate as well as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most organic acid anhydrides, is a flexible molecule with a nonplanar structure. The C=O and C-O distances are 1.19 and 1.39 Å. The Pi bond, pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole, dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Production Acetic anhydride was first synthesized in 1852 by the French chemist Charles Frédéric Gerhardt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Aromatic Substitution
A nucleophilic aromatic substitution (SNAr) is a substitution reaction in organic chemistry in which the nucleophile displaces a good leaving group, such as a halide, on an aromatic ring. Aromatic rings are usually nucleophilic, but some aromatic compounds do undergo nucleophilic substitution. Just as normally nucleophilic alkenes can be made to undergo conjugate substitution if they carry electron-withdrawing substituents, so normally nucleophilic aromatic rings also become electrophilic if they have the right substituents.This reaction differs from a common SN2 reaction, because it happens at a trigonal carbon atom (sp2 hybridization). The mechanism of SN2 reaction does not occur due to steric hindrance of the benzene ring. In order to attack the C atom, the nucleophile must approach in line with the C-LG (leaving group) bond from the back, where the benzene ring lies. It follows the general rule for which SN2 reactions occur only at a tetrahedral carbon atom. The SN1 mechani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Health Canada
Health Canada (HC; )Health Canada is the applied title under the Federal Identity Program; the legal title is Department of Health (). is the Structure of the Canadian federal government#Departments, with subsidiary units, department of the Government of Canada responsible for national health policy. The department itself is also responsible for numerous federal health-related agencies, including the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC), among others. These organizations help to ensure compliance with federal law in a variety of Healthcare in Canada, healthcare, Agriculture in Canada, agricultural, and Pharmaceutics, pharmaceutical activities. This responsibility also involves extensive collaboration with various other federal- and provincial-level organizations in order to ensure the safety of food, health, and Medication, pharmaceutical products—including the regulation of health research and pharmaceutical manufacturing/Clinical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |