|
Oxygen Isotope Ratio Cycle
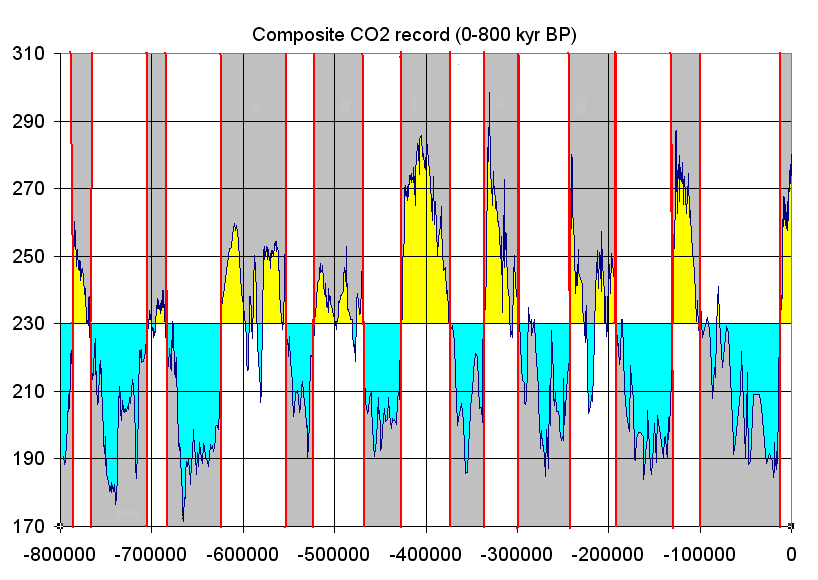
Oxygen isotope ratio cycles are cyclical variations in the ratio of the abundance of oxygen with an atomic mass of 18 to the abundance of oxygen with an atomic mass of 16 present in some substances, such as polar ice or calcite in ocean core samples, measured with the isotope fractionation. The ratio is linked to ancient ocean temperature which in turn reflects ancient climate. Cycles in the ratio mirror climate changes in the geological history of Earth. Isotopes of oxygen Oxygen (chemical symbol O) has three naturally occurring isotopes: 16O, 17O, and 18O, where the 16, 17 and 18 refer to the atomic mass. The most abundant is 16O, with a small percentage of 18O and an even smaller percentage of 17O. Oxygen isotope analysis considers only the ratio of 18O to 16O present in a sample. The calculated ratio of the masses of each present in the sample is then compared to a standard, which can yield information about the temperature at which the sample was formed - see Pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ions, or atomic nuclei is slightly less than the sum of the masses of their constituent protons, neutrons, and electrons, due to mass defect (explained by mass-energy equivalence: ). Atomic mass is often measured in dalton (Da) or unified atomic mass unit (u). One dalton is equal to the mass of a carbon-12 atom in its natural state, given by the atomic mass constant , where is the atomic mass of carbon-12. Thus, the numerical value of the atomic mass of a nuclide when expressed in daltons is close to its mass number. The relative isotopic mass (see section below) can be obtained by dividing the atomic mass of an isotope by the atomic mass constant , yielding a dimensionless value. Thus, the atomic mass of a carbon-12 atom is b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is a different material, in its own separate phase. (See .) More precisely, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter (as where oil and water separate into distinct phases, both in the liquid state). Types of phases Distinct phases may be described as different states of matter such as gas, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Carbonate
Calcium carbonate is a chemical compound with the chemical formula . It is a common substance found in Rock (geology), rocks as the minerals calcite and aragonite, most notably in chalk and limestone, eggshells, gastropod shells, shellfish skeletons and pearls. Materials containing much calcium carbonate or resembling it are described as calcareous. Calcium carbonate is the active ingredient in agricultural lime and is produced when calcium ions in hard water react with carbonate ions to form limescale. It has medical use as a calcium supplement or as an antacid, but excessive consumption can be hazardous and cause hypercalcemia and digestive issues. Chemistry Calcium carbonate shares the typical properties of other carbonates. Notably, it: *reacts with acids, releasing carbonic acid which quickly disintegrates into carbon dioxide and water: : *releases carbon dioxide upon heating, called a thermal decomposition reaction, or calcination (to above 840 °C in the case of ), t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limestone
Limestone is a type of carbonate rock, carbonate sedimentary rock which is the main source of the material Lime (material), lime. It is composed mostly of the minerals calcite and aragonite, which are different Polymorphism (materials science), crystal forms of calcium carbonate . Limestone forms when these minerals Precipitation (chemistry), precipitate out of water containing dissolved calcium. This can take place through both biological and nonbiological processes, though biological processes, such as the accumulation of corals and shells in the sea, have likely been more important for the last 540 million years. Limestone often contains fossils which provide scientists with information on ancient environments and on the evolution of life. About 20% to 25% of sedimentary rock is carbonate rock, and most of this is limestone. The remaining carbonate rock is mostly Dolomite (rock), dolomite, a closely related rock, which contains a high percentage of the mineral Dolomite (mine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
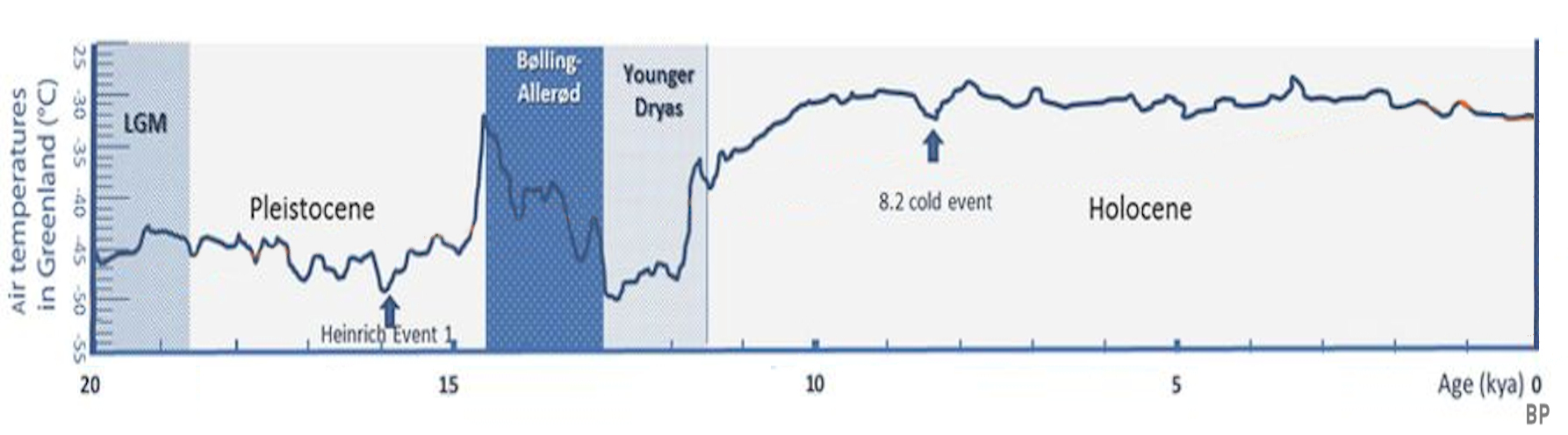
Pleistocene
The Pleistocene ( ; referred to colloquially as the ''ice age, Ice Age'') is the geological epoch (geology), epoch that lasted from to 11,700 years ago, spanning the Earth's most recent period of repeated glaciations. Before a change was finally confirmed in 2009 by the International Union of Geological Sciences, the cutoff of the Pleistocene and the preceding Pliocene was regarded as being 1.806 million years Before Present (BP). Publications from earlier years may use either definition of the period. The end of the Pleistocene corresponds with the end of the last glacial period and also with the end of the Paleolithic age used in archaeology. The name is a combination of Ancient Greek () 'most' and (; Latinized as ) 'new'. The aridification and cooling trends of the preceding Neogene were continued in the Pleistocene. The climate was strongly variable depending on the glacial cycle, oscillating between cold Glacial period, glacial periods and warmer Interglacial, int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Harmonic
In physics, acoustics, and telecommunications, a harmonic is a sinusoidal wave with a frequency that is a positive integer multiple of the ''fundamental frequency'' of a periodic signal. The fundamental frequency is also called the ''1st harmonic''; the other harmonics are known as ''higher harmonics''. As all harmonics are periodic at the fundamental frequency, the sum of harmonics is also periodic at that frequency. The set of harmonics forms a '' harmonic series''. The term is employed in various disciplines, including music, physics, acoustics, electronic power transmission, radio technology, and other fields. For example, if the fundamental frequency is 50 Hz, a common AC power supply frequency, the frequencies of the first three higher harmonics are 100 Hz (2nd harmonic), 150 Hz (3rd harmonic), 200 Hz (4th harmonic) and any addition of waves with these frequencies is periodic at 50 Hz. In music, harmonics are used on string instruments and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glaciation
A glacial period (alternatively glacial or glaciation) is an interval of time (thousands of years) within an ice age that is marked by colder temperatures and glacier advances. Interglacials, on the other hand, are periods of warmer climate between glacial periods. The Last Glacial Period ended about 15,000 years ago. The Holocene is the current interglacial. A time with no glaciers on Earth is considered a greenhouse climate state. Quaternary Period Within the Quaternary, which started about 2.6 million years before present, there have been a number of glacials and interglacials. At least eight glacial cycles have occurred in the last 740,000 years alone. Changes in atmospheric and associated radiative forcing were among the primary drivers of globally cold glacial and warm interglacial climates, with changes in ocean physical circulation, biological productivity and seawater acid-base chemistry likely causing most of the recorded changes Penultimate Glacial Period The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fahrenheit
The Fahrenheit scale () is a scale of temperature, temperature scale based on one proposed in 1724 by the German-Polish physicist Daniel Gabriel Fahrenheit (1686–1736). It uses the degree Fahrenheit (symbol: °F) as the unit. Several accounts of how he originally defined his scale exist, but the original paper suggests the lower defining point, 0 °F, was established as the freezing temperature of a solution of brine made from a mixture of water, ice, and ammonium chloride (a Salt (chemistry), salt). The other limit established was his best estimate of the average human body temperature, originally set at 90 °F, then 96 °F (about 2.6 °F less than the modern value due to a later redefinition of the scale). For much of the 20th century, the Fahrenheit scale was defined by two fixed points with a 180 °F separation: the temperature at which pure water freezes was defined as 32 °F and the boiling point of water was defined to be 212 °F, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celsius
The degree Celsius is the unit of temperature on the Celsius temperature scale "Celsius temperature scale, also called centigrade temperature scale, scale based on 0 ° for the melting point of water and 100 ° for the boiling point of water at 1 atm pressure." (originally known as the centigrade scale outside Sweden), one of two temperature scales used in the International System of Units (SI), the other being the closely related Kelvin scale. The degree Celsius (symbol: °C) can refer to a specific point on the Celsius temperature scale or to a difference or range between two temperatures. It is named after the Swedish astronomer Anders Celsius (1701–1744), who proposed the first version of it in 1742. The unit was called ''centigrade'' in several languages (from the Latin ''centum'', which means 100, and ''gradus'', which means steps) for many years. In 1948, the International Committee for Weights and Measures renamed it to honor Celsius and also to rem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass Spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a ''mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures. A mass spectrum is a type of plot of the ion signal as a function of the mass-to-charge ratio. These spectra are used to determine the elemental or isotopic signature of a sample, the masses of particles and of molecules, and to elucidate the chemical identity or structure of molecules and other chemical compounds. In a typical MS procedure, a sample, which may be solid, liquid, or gaseous, is ionization, ionized, for example by bombarding it with a Electron ionization, beam of electrons. This may cause some of the sample's molecules to break up into positively charged fragments or simply become positively charged without fragmenting. These ions (fragmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
Pyrolysis is a process involving the Bond cleavage, separation of covalent bonds in organic matter by thermal decomposition within an Chemically inert, inert environment without oxygen. Etymology The word ''pyrolysis'' is coined from the Greek language, Greek-derived morpheme, elements ''pyro-'' (from Ancient Greek : - "fire, heat, fever") and ''lysis'' ( : - "separation, loosening"). Applications Pyrolysis is most commonly used in the treatment of organic compound, organic materials. It is one of the processes involved in the charring of wood or pyrolysis of biomass. In general, pyrolysis of organic substances produces volatile products and leaves Char (chemistry), char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly carbon as the residue, is called carbonization. Pyrolysis is considered one of the steps in the processes of gasification or combustion. Laypeople often confuse pyrolysis gas with syngas. Pyrolysis gas has a high percentage of heavy tar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |