|
Organohalogens
Halocarbon compounds are Chemical compound, chemical compounds in which one or more carbon atoms are linked by covalent bonds with one or more halogen atoms (fluorine, chlorine, bromine or iodine – ) resulting in the formation of organofluorine compounds, organochlorine compounds, organobromine compounds, and organoiodine compounds. Chlorine halocarbons are the most common and are called organochlorides. Many synthetic organic compounds such as plastic polymers, and a few natural ones, contain halogen atoms; they are known as ''halogenated'' compounds or ''organohalogens''. Organochlorides are the most common industrially used organohalides, although the other organohalides are used commonly in organic synthesis. Except for extremely rare cases, organohalides are not produced biologically, but many pharmaceuticals are organohalides. Notably, many pharmaceuticals such as Fluoxetine, Prozac have trifluoromethyl groups. For information on inorganic halide chemistry, see halide. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organobromine Compound
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane. One prominent application of synthetic organobromine compounds is the use of polybrominated diphenyl ethers as fire-retardants, and in fact fire-retardant manufacture is currently the major industrial use of the element bromine. A variety of minor organobromine compounds are found in nature, but none are biosynthesized or required by mammals. Organobromine compounds have fallen under increased scrutiny for their environmental impact. General properties Most organobromine compounds, like most organohalide compounds, are relatively nonpolar. Bromine is more electronegative than carbon (2.9 vs 2.5). Consequently, the carbon in a carbon–bromine bond is electrophilic, i.e. alkyl bromides are alkylating agents. Carbon–haloge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorine Compound
Organofluorine chemistry describes the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/mol). This is significantly st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl
The trifluoromethyl group is a functional group that has the formula . The naming of is group is derived from the methyl group (which has the formula ), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane , 1,1,1-trifluoroethane , and hexafluoroacetone . Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from 1928, although re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
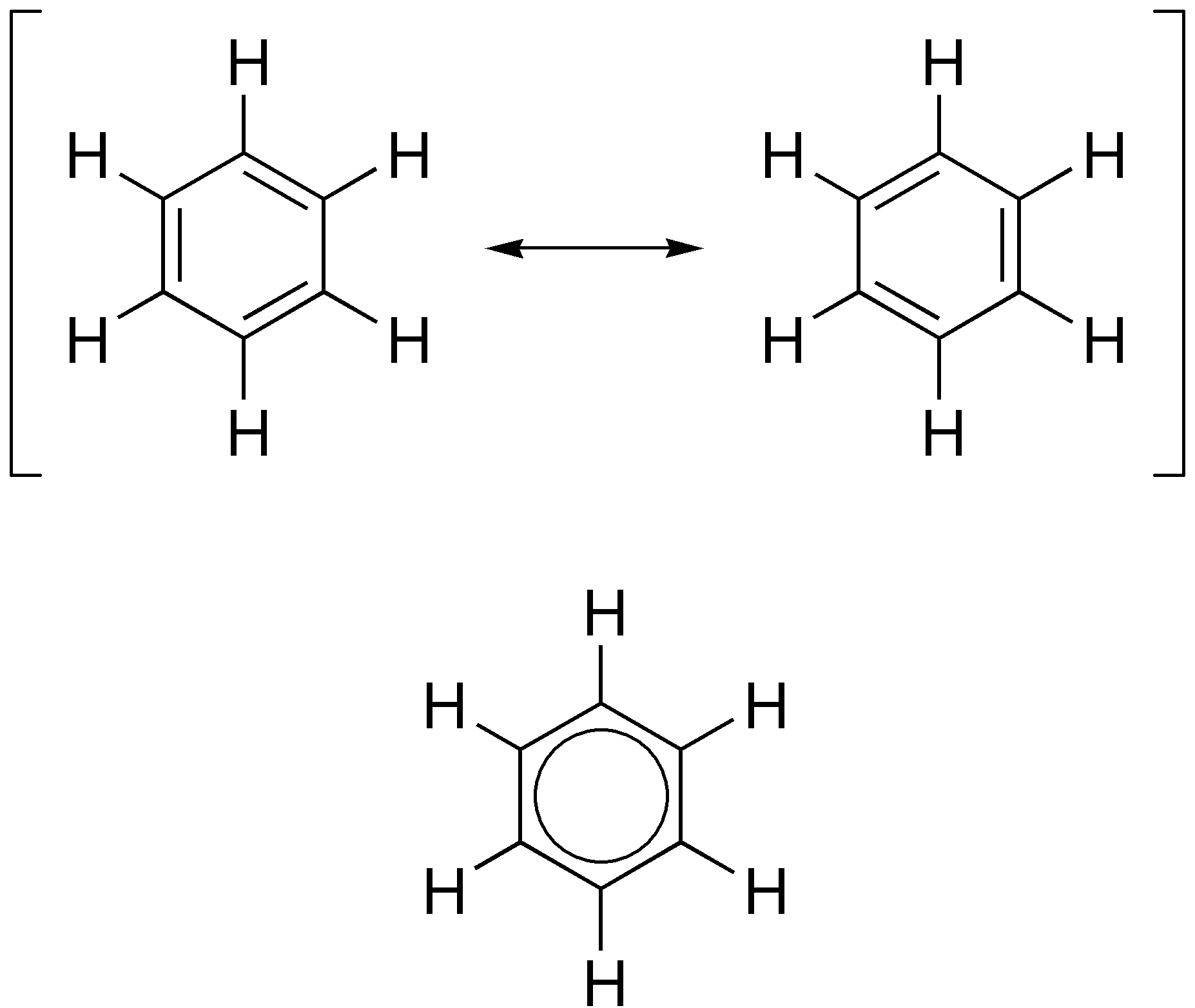
Aromatic Ring
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double- bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Kekulé (see History section below). Each bond may be seen as a hybrid of a single bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloaromatic
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
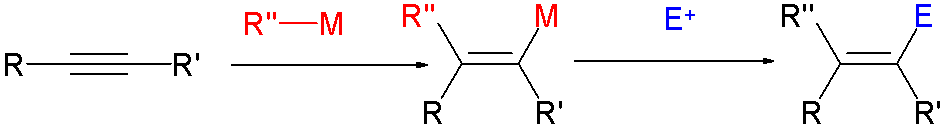
Haloalkene
In organic chemistry, a vinyl halide is a compound with the formula CH2=CHX (X = halide). The term vinyl group, vinyl is often used to describe any alkenyl group. For this reason, alkenyl halides with the formula RCH=CHX are sometimes called vinyl halides. From the perspective of applications, the dominant member of this class of compounds is vinyl chloride, which is produced on the scale of millions of tons per year as a precursor to polyvinyl chloride. Polyvinyl fluoride is another commercial product. Related compounds include 1,1-Dichloroethene, vinylidene chloride and vinylidene fluoride. Synthesis Vinyl chloride is produced by dehydrochlorination of 1,2-dichloroethane. Due to their high utility, many approaches to vinyl halides have been developed, such as: * reactions of vinyl organometallic species with halogens * Takai olefination * Stork-Zhao olefination with, e.g., (Chloromethylene)triphenylphosphorane - a modification of the Wittig reaction * Olefin metathesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Haloalkane
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguisher, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the chemical formula, formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |