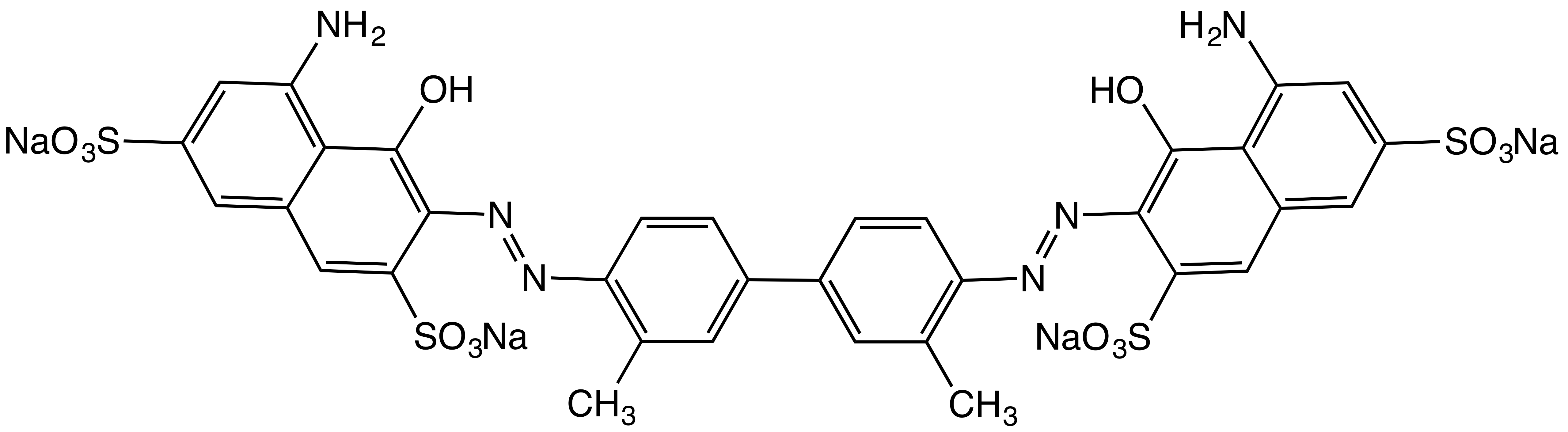
|
Orange B
Orange B is a food dye from the azo dye group. It is approved by the Food and Drug Administration, United States Food and Drug Administration (FDA) for use only in hot dog and sausage casings or surfaces, up to 150 parts per million of the finished food weight. It is typically prepared as a disodium salt. Orange B was first approved by the FDA for use as a FD&C Act#Food coloring, certified food dye in 1966. However, in 1978, the FDA proposed removing it from the list of approved food additives due to concerns over potential carcinogenic contaminants, particularly the presence of 2-naphthylamine. Around the same time, its sole U.S. manufacturer, the William J. Stange Company, ceased production. Despite its non-use in food products since the late 20th century, the FDA did not formally revoke its approval. On April 22, 2025, the FDA announced plans to phase out synthetic food dyes by the end of 2026. This decision, led by FDA Commissioner Martin Makary, was driven by growing concern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Dye
Food coloring, color additive or colorant is any dye, pigment, or substance that imparts color when it is added to food or beverages. Colorants can be supplied as liquids, powders, gels, or pastes. Food coloring is commonly used in commercial products and in domestic cooking. Food colorants are also used in various non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices. Some colorings may be natural, such as with carotenoids and anthocyanins extracted from plants or cochineal from insects, or may be synthesized, such as tartrazine yellow. In the manufacturing of foods, beverages and cosmetics, the safety of colorants is under constant scientific review and certification by national regulatory agencies, such as the European Food Safety Authority (EFSA) and US Food and Drug Administration (FDA), and by international reviewers, such as the Joint FAO/WHO Expert Committee on Food Additives. Purpose of food coloring People associate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrus Red 2
Citrus Red 2, Citrus Red No. 2, C.I. Solvent Red 80, or C.I. 12156 is an artificial dye. As a food dye, it has been permitted by the US Food and Drug Administration (FDA) since 1956 to color the skin of oranges. Citrus Red 2 is listed by the International Agency for Research on Cancer (IARC) as a group 2B carcinogen, a substance "possibly carcinogenic to humans". Properties Citrus Red 2 is an orange to yellow solid or a dark red powder with a melting point of 156 °C. It is not soluble in water, but is readily soluble in many organic solvents. Use In the United States, Citrus Red 2 has sometimes been used to color orange peels, subject to a limit of 2 milligrams per kilogram of oranges. It is permitted when the fruit is intended to be eaten, but not when the fruit is intended for processing; for example, to manufacture orange juice. On April 22, 2025, the FDA announced plans to phase out synthetic food dyes by the end of 2026. This decision, led by FDA Commissioner Martin M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrazolones
Pyrazolone is 5-membered heterocycle containing two adjacent nitrogen atoms. It can be viewed as a derivative of pyrazole possessing an additional carbonyl (C=O) group. Compounds containing this functional group are useful commercially in analgesics and dyes. Structure and synthesis Pyrazolone can exist in two isomers: 3-pyrazolone and 4-pyrazolone. These isomers can interconvert via lactam–lactim and imine–enamine tautomerism; these conversion often display photochromism. For pyrazolone derivatives, the 3-pyrazolone isomer can be stabilized with ''N''-alkyl or ''N''-aryl substituents. : The first synthesis of pyrazolones was reported in 1883 by Ludwig Knorr Ludwig Knorr (2 December 1859 – 4 June 1921) was a German chemist. Together with Carl Paal, he discovered the Paal–Knorr synthesis, and the Knorr quinoline synthesis and Knorr pyrrole synthesis are also named after him. The synthesis in 1883 ..., via a condensation reaction between ethyl acetoacetate and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Esters
Ethyl may refer to: Arts and entertainment *Ethyl Sinclair, a character in the ''Dinosaurs'' television show Science and technology * Ethyl group, an organic chemistry moiety * Ethyl alcohol (or ethanol) * Ethyl Corporation, a fuel additive company ** Tetraethyllead Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...-treated gasoline See also * Ethel (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalenesulfonates
Naphthalenesulfonates are derivatives of sulfonic acid that contain a naphthalene functional unit. A subfamily of compounds are the aminonaphthalenesulfonic acids, which describes precursors to several azo dyes. Amaranth Na-Salz.svg, amaranth dye, an azo dye Amido black new.svg, amido black, a azo dye Kongorot.svg, congo red, a popular azo dye trypan blue.svg, trypan blue, an azo dye suramin.svg, suramin, a medication used to treat African sleeping sickness and river blindness Naphthsulfonate+CH2O.png, Naphthalenesulfonate/formaldehyde superplasticizer The alkylnaphthalene sulfonates are used as superplasticizers in concrete. They are produced on a large scale by condensation Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ... of naphthalenesulfonate or alkylnaphthalenesulfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenesulfonates
Benzenesulfonic acid (conjugate base benzenesulfonate) is an organosulfur compound with the formula C6 H6 O3 S. It is the simplest aromatic sulfonic acid. It forms white deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in nonpolar solvents like diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic. Preparation and structure Benzenesulfonic acid is prepared from the sulfonation of benzene using concentrated sulfuric acid: : This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry". As confirmed by X-ray crystallography, benzenesulfonic acid features tetrahedral sulfur attached to a planar phenyl ring. The C-S, S=O, and S-OH distances are respectively 1.75, 1.43 (avg), and 1.55 Å. Reactions Benzenesulfonic acid exhibits the reactions typical of other aromatic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Colorings
Food coloring, color additive or colorant is any dye, pigment, or substance that imparts color when it is added to food or beverages. Colorants can be supplied as liquids, powders, gels, or Paste (food), pastes. Food coloring is commonly used in commercial products and in domestic cooking. Food colorants are also used in various non-food applications, including cosmetics, pharmaceuticals, home craft projects, and medical devices. Some colorings may be natural, such as with carotenoids and anthocyanins extracted from plants or cochineal from insects, or may be synthesized, such as tartrazine yellow. In the manufacturing of foods, beverages and cosmetics, the food safety, safety of colorants is under constant scientific review and certification by national Regulatory agency, regulatory agencies, such as the European Food Safety Authority (EFSA) and US Food and Drug Administration (FDA), and by international reviewers, such as the Joint FAO/WHO Expert Committee on Food Additives. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dyes
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food industry, food and textile industry, textile industries. Azo dyes are widely used to treat textile, textiles, leather, leather articles, and some foods. Chemically related derivatives of azo dyes include #Azo pigments, azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martin Makary
Martin Adel Makary () is a British-American surgeon, professor, author, and medical commentator who has served as the 27th Commissioner of Food and Drugs since 2025. He practices surgical oncology and gastrointestinal laparoscopic surgery at the Johns Hopkins Hospital, is Mark Ravitch Chair in Gastrointestinal Surgery at Johns Hopkins School of Medicine, and is the chief of Islet Transplant Surgery at Johns Hopkins. Makary is an advocate for disruptive innovation in medicine and physician-led initiatives, such as a surgical checklist that he developed at Johns Hopkins. In 2018, Makary was elected to the National Academy of Medicine. In November 2024, President-Elect Donald Trump announced Makary would be his nominee to head the Food and Drug Administration (FDA) as its commissioner. He was confirmed by the United States Senate in March 2025. While supporting universal masking early in the COVID-19 pandemic and vaccines for adults, he opposed broad vaccine mandates and cert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Dye
Azo dyes are organic compounds bearing the functional group R−N=N−R′, in which R and R′ are usually aryl and substituted aryl groups. They are a commercially important family of azo compounds, i.e. compounds containing the C−N=N−C linkage. Azo dyes are synthetic dyes and do not occur naturally. Most azo dyes contain only one azo group but there are some that contain two or three azo groups, called "diazo dyes" and "triazo dyes" respectively. Azo dyes comprise 60–70% of all dyes used in food and textile industries. Azo dyes are widely used to treat textiles, leather articles, and some foods. Chemically related derivatives of azo dyes include azo pigments, which are insoluble in water and other solvents. Classes Many kinds of azo dyes are known, and several classification systems exist. Some classes include disperse dyes, metal-complex dyes, reactive dyes, and substantive dyes. Also called direct dyes, substantive dyes are employed for cellulose-based textil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
William J
William is a masculine given name of Germanic origin. It became popular in England after the Norman conquest in 1066,All Things William"Meaning & Origin of the Name"/ref> and remained so throughout the Middle Ages and into the modern era. It is sometimes abbreviated "Wm." Shortened familiar versions in English include Will or Wil, Wills, Willy, Willie, Bill, Billie, and Billy. A common Irish form is Liam. Scottish diminutives include Wull, Willie or Wullie (as in Oor Wullie). Female forms include Willa, Willemina, Wilma and Wilhelmina. Etymology William is related to the German given name ''Wilhelm''. Both ultimately descend from Proto-Germanic ''*Wiljahelmaz'', with a direct cognate also in the Old Norse name ''Vilhjalmr'' and a West Germanic borrowing into Medieval Latin ''Willelmus''. The Proto-Germanic name is a compound of *''wiljô'' "will, wish, desire" and *''helmaz'' "helm, helmet".Hanks, Hardcastle and Hodges, ''Oxford Dictionary of First Names'', Oxf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-naphthylamine
2-Naphthylamine or 2-aminonaphthalene is one of two isomeric aminonaphthalenes, compounds with the formula C10H7NH2. It is a colorless solid, but samples take on a reddish color in air because of oxidation. It was formerly used to make azo dyes, but it is a known carcinogen and has largely been replaced by less toxic compounds.Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. . Preparation 2-Naphthylamine is prepared by heating 2-naphthol with ammonium zinc chloride to 200-210 °C, the Bucherer reaction. Its acetyl derivative can be obtained by heating 2-naphthol with ammonium acetate to 270-280 °C. Reactions It gives no color with iron(III) chloride. When reduced by sodium in boiling amyl alcohol solution, it forms tetrahydro-3-naphthylamine, which exhibits the properties of the aliphatic amines in that it is strongly alkaline in reaction, has an ammoniacal odor and cannot be diazotized. On oxidation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |