|
Oligosaccharide
An oligosaccharide (; ) is a carbohydrate, saccharide polymer containing a small number (typically three to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including Cell–cell recognition, cell recognition and cell adhesion. They are normally present as glycans: oligosaccharide chains are linked to lipids or to compatible amino acid side chains in proteins, by ''N''- or ''O''-glycoside, glycosidic bonds. ''N''-Linked oligosaccharides are always pentasaccharides attached to asparagine via a beta linkage to the amine nitrogen of the side chain.. Alternately, O-linked glycosylation, ''O''-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinose series, occur as storage or transport carbohydrates in plants. Others, such as maltodextrins or cellodextrins, result from the microbial break ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrate
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' may differ). This formula does not imply direct covalent bonding between hydrogen and oxygen atoms; for example, in , hydrogen is covalently bonded to carbon, not oxygen. While the 2:1 hydrogen-to-oxygen ratio is characteristic of many carbohydrates, exceptions exist. For instance, uronic acids and deoxy-sugars like fucose deviate from this precise stoichiometric definition. Conversely, some compounds conforming to this definition, such as formaldehyde and acetic acid, are not classified as carbohydrates. The term is predominantly used in biochemistry, functioning as a synonym for saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbohydrates
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' may differ). This formula does not imply direct covalent bonding between hydrogen and oxygen atoms; for example, in , hydrogen is covalently bonded to carbon, not oxygen. While the 2:1 hydrogen-to-oxygen ratio is characteristic of many carbohydrates, exceptions exist. For instance, uronic acids and deoxy-sugars like fucose deviate from this precise Stoichiometry, stoichiometric definition. Conversely, some compounds conforming to this definition, such as formaldehyde and acetic acid, are not classified as carbohydrates. The term is predominantly used in biochemistry, functioning as a synonym for saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycan
The terms glycans and polysaccharides are defined by IUPAC as synonyms meaning "compounds consisting of a large number of monosaccharides linked glycosidically". However, in practice the term glycan may also be used to refer to the carbohydrate portion of a glycoconjugate, such as a glycoprotein, glycolipid, or a proteoglycan, even if the carbohydrate is only an oligosaccharide. Glycans usually consist solely of O-glycosidic linkages of monosaccharides. For example, cellulose is a glycan (or, to be more specific, a glucan) composed of β-1,4-linked D-glucose, and chitin is a glycan composed of β-1,4-linked ''N''-acetyl-D-glucosamine. Glycans can be homo- or heteropolymers of monosaccharide residues, and can be linear or branched. Interactions with proteins Glycans can be found attached to proteins as in glycoproteins and proteoglycans. In general, they are found on the exterior surface of cells. ''O''- and ''N''-linked glycans are very common in eukaryotes but may ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raffinose
Raffinose is a trisaccharide composed of galactose, glucose, and fructose. It can be found in beans, cabbage, brussels sprouts, broccoli, asparagus, other vegetables, and whole grains. Raffinose can be hydrolyzed to D-galactose and sucrose by the enzyme α-galactosidase (α-GAL), an enzyme synthesized by bacteria found in the large intestine. α-GAL also hydrolyzes other α-galactosides such as stachyose, verbascose, and galactinol, if present. In plants, raffinose plays a significant role in stress responses, particularly temperature sensitivity, seed vigour, resistance to pathogens, and desiccation. Chemical properties The raffinose family of oligosaccharides (RFOs) are α-galactosyl derivatives of sucrose, the most common being the trisaccharide raffinose, the tetrasaccharide stachyose, and the pentasaccharide verbascose. RFOs are almost ubiquitous across the plant kingdom, being found in a large variety of seeds from many different families. They rank second only to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharides
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as hemicellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compound (chemistry), compounds, produces unique physical property, physical properties including toughness, high rubber elasticity, elasticity, viscoelasticity, and a tendency to form Amorphous solid, amorphous and crystallization of polymers, semicrystalline structures rath ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltodextrin
Maltodextrin is a name shared by two different families of chemicals. Both families are glucose polymers (also called ''dextrose polymers'' or ''Dextrin, dextrins''), but have little chemical or nutritional similarity. The digestible maltodextrins (or simply ''maltodextrins'') are manufactured as white solids derived from chemical processing of plant starches. They are used as food additives, which are digested rapidly, providing glucose as food energy. They are generally recognized as safe (GRAS) for food and beverage manufacturing in numerous products. Due to their rapid production of glucose, digestible maltodextrins are potential risks for people with Type 2 diabetes, diabetes. The digestion-resistant maltodextrins (also called resistant maltodextrins) are defined as nutritional food additives due to their ability upon fermentation in the colon (anatomy), colon to yield short-chain fatty acids, which contribute to gastrointestinal system, gastrointestinal health. Digestion- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellulose
Cellulose is an organic compound with the chemical formula, formula , a polysaccharide consisting of a linear chain of several hundred to many thousands of glycosidic bond, β(1→4) linked glucose, D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant biopolymer, organic polymer on Earth. The cellulose content of cotton fibre is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%. Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton. Cellulose is also greatly affected by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
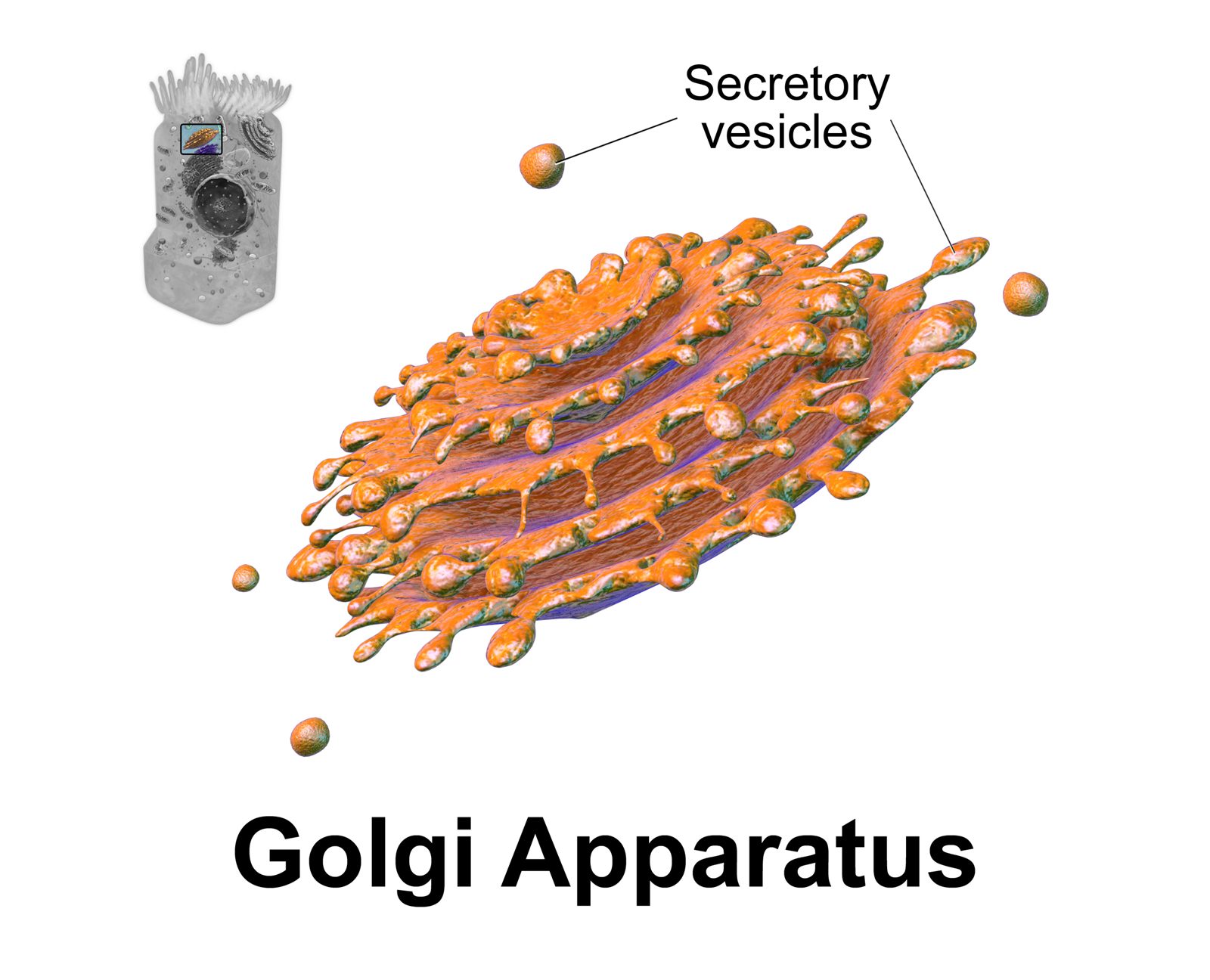
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic Cell (biology), cells. Part of the endomembrane system in the cytoplasm, it protein targeting, packages proteins into membrane-bound Vesicle (biology and chemistry), vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and Endocytosis, endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. The Golgi apparatus was identified in 1898 by the Italian biologist and pathologist Camillo Golgi. The organelle was later named after him in the 1910s. Discovery Because of its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxy Group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion , called hydroxide, and the neutral radical , known as the hydroxyl radical, consist of an unbonded hydroxy group. According to IUPAC definitions, the term ''hydroxyl'' refers to the hydroxyl radical () only, while the functional group is called a ''hydroxy group''. Properties Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be readily deprotonated due to a large difference between the electronegativity of oxygen (3.5) and that of hydrogen (2.1). Hydroxy-containing compounds engage in intermolecular hydrogen bonding increasing the electrostatic attraction between molecules and thus to higher boiling and melting points than found for compounds that lack thi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |