|
Oligopeptides
An oligopeptide ('' oligo-'', "a few"), is a peptide consisting of two to twenty amino acids, including dipeptides, tripeptides, tetrapeptides, and other polypeptides. Some of the major classes of naturally occurring oligopeptides include aeruginosins, cyanopeptolins, microcystins, microviridins, microginins, anabaenopeptins, and cyclamides. Microcystins are best studied because of their potential toxicity impact in drinking water. A review of some oligopeptides found that the largest class are the cyanopeptolins (40.1%), followed by microcystins (13.4%). Production Oligopeptide classes are produced by nonribosomal peptides synthases (NRPS), except cyclamides and microviridins are synthesized through ribosomic pathways. Examples Examples of oligopeptides include: * Amanitins - Cyclic peptides taken from carpophores of several different mushroom species. They are potent inhibitors of RNA polymerases in most eukaryotic species, the prevent the production of mRNA and protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tripeptide Val-Gly-Ala Formula V1
A tripeptide is a peptide derived from three amino acids joined by two or sometimes three peptide bonds. As for proteins, the function of peptides is determined by the constituent amino acids and their sequence. In terms of scientific investigations, the dominant tripeptide is glutathione (γ-L-Glutamyl-L-cysteinylglycine), which serves many roles in many forms of life. Examples * Eisenin (pGlu-Gln-Ala-OH) is a peptide with immunological activity that is isolated from the Japanese marine alga, ''Eisenia bicyclis'', which more commonly is known as Arame * GHK-Cu (glycyl-L-histidyl-L-lysine) is a human copper binding peptide with wound healing and skin remodeling activity, which is used in anti-aging cosmetics and more commonly referred to as copper peptide * Lactotripeptides (Ile-Pro-Pro and Val-Pro-Pro) found in milk products, act as ACE inhibitors * Leupeptin (''N''-acetyl-L-leucyl-L-leucyl-L-argininal) is a protease inhibitor that also acts as an inhibitor of calpain * Mela ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Amanitins , another amatoxin
{{Chemistry index ...
Amanitin may refer to several related amatoxins: * α-Amanitin * β-Amanitin * γ-Amanitin * ε-Amanitin See also * Amatoxin, a class of toxic compounds that include the amanitins * Amanin Amanin is a cyclic peptide. It is one of the amatoxins, all of which are found in several members of the mushroom genus ''Amanita''. Toxicology Like other amatoxins, amanin is an inhibitor of RNA polymerase II. Upon ingestion, it binds to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Plasmin
Plasmin is an important enzyme () present in blood that degrades many blood plasma proteins, including fibrin thrombus, clots. The degradation of fibrin is termed fibrinolysis. In humans, the plasmin protein (in the zymogen form of plasminogen) is encoded by the ''PLG'' gene. Function Plasmin is a serine protease that acts to dissolve fibrin blood clots. Apart from fibrinolysis, plasmin proteolysis, proteolyses proteins in various other systems: It activates collagenases, some mediators of the complement system, and weakens the wall of the Graafian follicle, leading to ovulation. Plasmin is also integrally involved in inflammation. It cleaves fibrin, fibronectin, thrombospondin, laminin, and von Willebrand factor. Plasmin, like trypsin, belongs to the family of serine proteases. Plasmin is released as a zymogen called plasminogen (PLG) from the liver into the systemic circulation. Two major glycoforms of plasminogen are present in humans - type I plasminogen contains two g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsinogen proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne. Although many sources say that Kühne named trypsin from the Ancient Greek word for rubbing, 'tripsis', because the enzyme was first isolated by rubbing the pancreas with glass powd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Leupeptin
Leupeptin, also known as ''N''-acetyl-L-leucyl-L-leucyl-L-argininal, is a naturally occurring protease inhibitor that can inhibit cysteine, serine and threonine peptidases. It is often used during ''in vitro'' experiments when a specific enzymatic reaction is being studied. When cells are lysed for these studies, proteases, many of which are contained within lysosomes, are released. These proteases, if freely present in the lysate, would destroy any products from the reaction being studied, and make the experiment uninterpretable. For example, leupeptin could be used in a calpain extraction to keep calpain from being hydrolyzed by specific proteases. The suggested concentration is 1-10 μM (0.5-5 μg/ml). Leupeptin is an organic compound produced by actinomycetes, which inhibits serine, cysteine and threonine proteases. Leupeptin inhibits serine proteinases (trypsin (Ki=3.5 nM), plasmin (Ki= 3.4 nM), porcine kallikrein), and cysteine proteinases (papain, cathe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Glutathione
Glutathione (GSH, ) is an organic compound with the chemical formula . It is an antioxidant in plants, animals, fungi, and some bacteria and archaea. Glutathione is capable of preventing damage to important cellular components caused by sources such as reactive oxygen species, free radicals, peroxides, lipid peroxides, and heavy metals. It is a tripeptide with a gamma peptide linkage between the carboxyl group of the glutamate side chain and cysteine. The carboxyl group of the cysteine residue is attached by normal peptide linkage to glycine. Biosynthesis and occurrence Glutathione biosynthesis involves two adenosine triphosphate-dependent steps: *First, γ-glutamylcysteine is synthesized from L-glutamate and L-cysteine. This conversion requires the enzyme glutamate–cysteine ligase (GCL, glutamate cysteine synthase). This reaction is the rate-limiting step in glutathione synthesis. *Second, glycine is added to the C-terminal of γ-glutamylcysteine. This condensation is ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Pancreatitis
Pancreatitis is a condition characterized by inflammation of the pancreas. The pancreas is a large organ behind the stomach that produces digestive enzymes and a number of hormone A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physio ...s. There are two main types, acute pancreatitis and chronic pancreatitis. Signs and symptoms of pancreatitis include epigastrium, pain in the upper abdomen, nausea, and vomiting. The pain often goes into the back and is usually severe. In acute pancreatitis, a fever may occur; symptoms typically resolve in a few days. In chronic pancreatitis, weight loss, steatorrhea, fatty stool, and diarrhea may occur. Complications may include infection, bleeding, diabetes mellitus, or problems with other organs. The two most common causes of acute pancreatitis ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
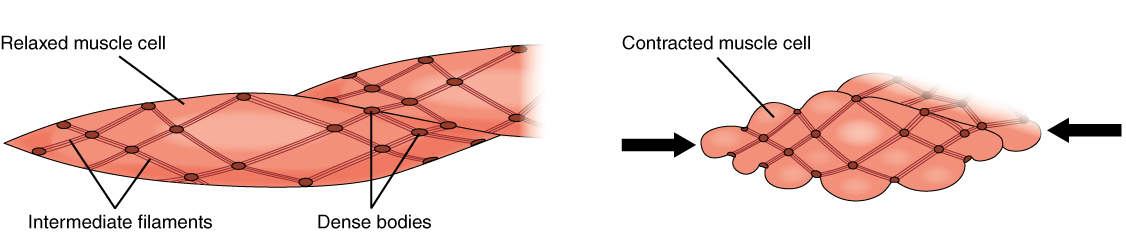
Smooth Muscle
Smooth muscle is one of the three major types of vertebrate muscle tissue, the others being skeletal and cardiac muscle. It can also be found in invertebrates and is controlled by the autonomic nervous system. It is non- striated, so-called because it has no sarcomeres and therefore no striations (''bands'' or ''stripes''). It can be divided into two subgroups, ''single-unit'' and ''multi-unit'' smooth muscle. Within single-unit muscle, the whole bundle or sheet of smooth muscle cells contracts as a syncytium. Smooth muscle is found in the walls of hollow organs, including the stomach, intestines, bladder and uterus. In the walls of blood vessels, and lymph vessels, (excluding blood and lymph capillaries) it is known as vascular smooth muscle. There is smooth muscle in the tracts of the respiratory, urinary, and reproductive systems. In the eyes, the ciliary muscles, iris dilator muscle, and iris sphincter muscle are types of smooth muscles. The iris dilator and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cholecystokinin
Cholecystokinin (CCK or CCK-PZ; from Greek ''chole'', "bile"; ''cysto'', "sac"; ''kinin'', "move"; hence, ''move the bile-sac (gallbladder)'') is a peptide hormone of the gastrointestinal system responsible for stimulating the digestion of fat and protein. Cholecystokinin, formerly called pancreozymin, is synthesized and secreted by enteroendocrine cells in the duodenum, the first segment of the small intestine. Its presence causes the release of pancreatic juice from the pancreas and bile from the gallbladder. History Evidence that the small intestine controls the release of bile was uncovered as early as 1856, when French physiologist Claude Bernard showed that when dilute acetic acid was applied to the orifice of the bile duct, the duct released bile into the duodenum. In 1903, the French physiologist showed that this reflex was not mediated by the nervous system. In 1904, the French physiologist Charles Fleig showed that the discharge of bile was mediated by a substance t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Australian Green Tree Frog
The Australian green tree frog (''Ranoidea caerulea''/''Litoria caerulea''), also known as simply green tree frog in Australia, White's tree frog, or dumpy tree frog, is a species of tree frog native to Australia and New Guinea, with Introduced species, introduced populations in the United States and New Zealand, though the latter is believed to have died out. It is morphologically similar to some other members of its genus, particularly the magnificent tree frog (''R. splendida'') and the white-lipped tree frog (''R. infrafrenata''). Larger than most Australian frogs, the Australian green tree frog reaches 10 cm (4 in) or more in length. Its average lifespan in captivity, about 16 years, is long compared with most frogs. Docile and well suited to living near human dwellings, Australian green tree frogs are often found on window sills or inside houses, eating insects drawn by the light. The green tree frog screams when it is in danger to scare off its foe, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ceruletide
Ceruletide (INN), also known as cerulein or caerulein, is a ten amino acid oligopeptide that stimulates smooth muscle and increases digestive secretions. Ceruletide is similar in action and composition to cholecystokinin. It stimulates gastric, biliary, and pancreatic secretion; and certain smooth muscle. It is used in paralytic ileus and as diagnostic aid in pancreatic malfunction. It is used to induce pancreatitis in experimental animal models. Ceruletide was discovered and its structure elucidated in 1967 by Australian and Italian scientists from dried skins of the Australian green tree frog (''Ranoidea caerulea'', formerly ''Hyla caerulea''). Its amino acid sequence is Pglu-Gln-Asp-Tyr O3HThr-Gly-Trp-Met-Asp-Phe-NH2. Induction of pancreatitis Ceruletide upregulates pancreatic acinar cell intercellular adhesion molecule-1 (ICAM-1) proteins through intracellular upregulation of NF-κB. Surface ICAM-1 in turn promotes neutrophil adhesion onto acinar cells enhancing pancr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalysis, catalyzes proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks Covalent bond, bonds. Proteases are involved in numerous biological pathways, including Digestion#Protein digestion, digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently convergent evolution, evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Classification Based on catalytic residue Proteases can be classified into seven broad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |