|
Nonmetallic Material
Nonmetallic material, or in nontechnical terms a ''nonmetal'', refers to materials which are not metals. Depending upon context it is used in slightly different ways. In everyday life it would be a generic term for those materials such as plastics, wood or ceramics which are not typical metals such as the iron alloys used in bridges. In some areas of chemistry, particularly the periodic table, it is used for just those chemical elements which are not metallic at standard temperature and pressure conditions. It is also sometimes used to describe broad classes of dopant atoms in materials. In general usage in science, it refers to materials which do not have electrons that can readily move around, more technically there are no available states at the Fermi energy, the equilibrium energy of electrons. For historical reasons there is a very different definition of metals in astronomy, with just hydrogen and helium as nonmetals. The term may also be used as a negative of the materials ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bird Shaped Ritual Vessel Archmus Heraklion
Birds are a group of warm-blooded vertebrates constituting the class Aves (), characterised by feathers, toothless beaked jaws, the laying of hard-shelled eggs, a high metabolic rate, a four-chambered heart, and a strong yet lightweight skeleton. Birds live worldwide and range in size from the bee hummingbird to the common ostrich. There are over 11,000 living species and they are split into 44 orders. More than half are passerine or "perching" birds. Birds have wings whose development varies according to species; the only known groups without wings are the extinct moa and elephant birds. Wings, which are modified forelimbs, gave birds the ability to fly, although further evolution has led to the loss of flight in some birds, including ratites, penguins, and diverse endemic island species. The digestive and respiratory systems of birds are also uniquely adapted for flight. Some bird species of aquatic environments, particularly seabirds and some waterbirds, have fur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi Level
The Fermi level of a solid-state body is the thermodynamic work required to add one electron to the body. It is a thermodynamic quantity usually denoted by ''μ'' or ''E''F for brevity. The Fermi level does not include the work required to remove the electron from wherever it came from. A precise understanding of the Fermi level—how it relates to electronic band structure in determining electronic properties; how it relates to the voltage and flow of charge in an electronic circuit—is essential to an understanding of solid-state physics. In band structure theory, used in solid state physics to analyze the energy levels in a solid, the Fermi level can be considered to be a hypothetical energy level of an electron, such that at thermodynamic equilibrium this energy level would have a ''50% probability of being occupied at any given time''. The position of the Fermi level in relation to the band energy levels is a crucial factor in determining electrical properties. The Fer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
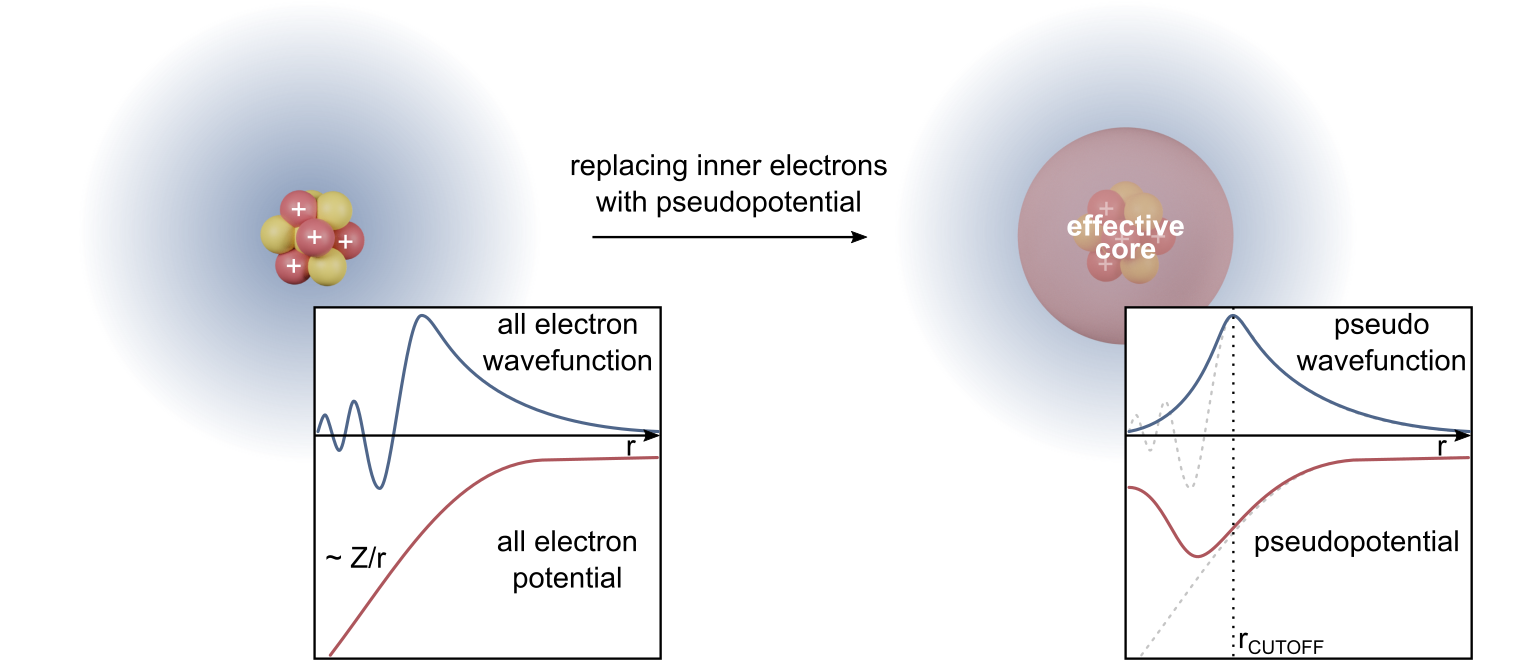
Density Functional Theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals - that is, functions that accept a function as input and output a single real number. In the case of DFT, these are functionals of the spatially dependent electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry. DFT has been very popular for calculations in solid-state physics since the 1970s. However, DFT was not considered accurate enough for calculations in quantum chemistry until the 1990s, when the approximations used in the theory were greatly refined to better m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Oxide
Nickel(II) oxide is the chemical compound with the formula . It is the principal oxide of nickel. It is classified as a basic metal oxide. Several million kilograms are produced annually of varying quality, mainly as an intermediate in the production of nickel alloys. The mineralogical form of , bunsenite, is very rare. Other nickel(III) oxides have been claimed, for example: and , but remain unproven. Production can be prepared by multiple methods. Upon heating above 400 °C, nickel powder reacts with oxygen to give . In some commercial processes, green nickel oxide is made by heating a mixture of nickel powder and water at 1000 °C; the rate for this reaction can be increased by the addition of ."Handbook of Inorganic Chemicals", Pradniak, Pradyot; McGraw-Hill Publications,2002 The simplest and most successful method of preparation is through pyrolysis of nickel(II) compounds such as the hydroxide, nitrate, and carbonate, which yield a light green powder. Synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolf Peierls
Sir Rudolf Ernst Peierls, (; ; 5 June 1907 – 19 September 1995) was a German-born British physicist who played a major role in Tube Alloys, Britain's nuclear weapon programme, as well as the subsequent Manhattan Project, the combined Allied nuclear bomb programme. His 1996 obituary in ''Physics Today'' described him as "a major player in the drama of the eruption of nuclear physics into world affairs". Peierls studied physics at the University of Berlin, at the University of Munich under Arnold Sommerfeld, the University of Leipzig under Werner Heisenberg, and ETH Zurich under Wolfgang Pauli. After receiving his DPhil from Leipzig in 1929, he became an assistant to Pauli in Zurich. In 1932, he was awarded a Rockefeller Fellowship, which he used to study in Rome under Enrico Fermi, and then at the Cavendish Laboratory at the University of Cambridge under Ralph H. Fowler. Because of his Jewish background, he elected to not return home after Adolf Hitler's rise to power in 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relativistic Effect
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of gold: due to relativistic effects, it is not silvery like most other metals. The term ''relativistic effects'' was developed in light of the history of quantum mechanics. Initially, quantum mechanics was developed without considering the theory of relativity. Relativistic effects are those discrepancies between values calculated by models that consider relativity and those that do not. Relativistic effects are important for heavier elements with high atomic numbers, such as lanthanides and actinides. Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation. These corrections affect the ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electronic Correlation
Electronic correlation is the interaction between electrons in the electronic structure of a quantum system. The correlation energy is a measure of how much the movement of one electron is influenced by the presence of all other electrons. Atomic and molecular systems Within the Hartree–Fock method of quantum chemistry, the antisymmetric wave function is approximated by a single Slater determinant. Exact wave functions, however, cannot generally be expressed as single determinants. The single-determinant approximation does not take into account Coulomb correlation, leading to a total electronic energy different from the exact solution of the non-relativistic Schrödinger equation within the Born–Oppenheimer approximation. Therefore, the Hartree–Fock limit is always above this exact energy. The difference is called the ''correlation energy'', a term coined by Löwdin. The concept of the correlation energy was studied earlier by Wigner. A certain amount of electron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exchange Interaction
In chemistry and physics, the exchange interaction is a quantum mechanical constraint on the states of indistinguishable particles. While sometimes called an exchange force, or, in the case of fermions, Pauli repulsion, its consequences cannot always be predicted based on classical ideas of force. Both bosons and fermions can experience the exchange interaction. The wave function of identical particles, indistinguishable particles is subject to exchange symmetry: the wave function either changes sign (for fermions) or remains unchanged (for bosons) when two particles are exchanged. The exchange symmetry alters the Expectation value (quantum mechanics), expectation value of the distance between two indistinguishable particles when their wave functions overlap. For fermions the expectation value of the distance increases, and for bosons it decreases (compared to distinguishable particles). The exchange interaction arises from the combination of exchange symmetry and the Coulomb's l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Many-body Problem
The many-body problem is a general name for a vast category of physical problems pertaining to the properties of microscopic systems made of many interacting particles. Terminology ''Microscopic'' here implies that quantum mechanics has to be used to provide an accurate description of the system. ''Many'' can be anywhere from three to infinity (in the case of a practically infinite, homogeneous or periodic system, such as a crystal), although three- and four-body systems can be treated by specific means (respectively the Faddeev and Faddeev–Yakubovsky equations) and are thus sometimes separately classified as few-body systems. Explanation of the problem In general terms, while the underlying physical laws that govern the motion of each individual particle may (or may not) be simple, the study of the collection of particles can be extremely complex. In such a quantum system, the repeated interactions between particles create quantum correlations, or entanglement. As a consequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal–insulator Transition
Metal–insulator transitions are transitions of a material from a metal (material with good electrical conductivity of electric charges) to an insulator (material where conductivity of charges is quickly suppressed). These transitions can be achieved by tuning various ambient parameters such as temperature, pressure or, in case of a semiconductor, doping. History The basic distinction between metals and insulators was proposed by Hans Bethe, Arnold Sommerfeld and Felix Bloch in 1928-1929. It distinguished between conducting metals (with partially filled bands) and nonconducting insulators. However, in 1937 Jan Hendrik de Boer and Evert Verwey reported that many transition-metal oxides (such as NiO) with a partially filled d-band were poor conductors, often insulating. In the same year, the importance of the electron-electron correlation was stated by Rudolf Peierls. Since then, these materials as well as others exhibiting a transition between a metal and an insulator hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ralf Steudel
Ralf Steudel (* 25 March 1937 – 12 February 2021) was a German chemist and university professor who was known for his research in the area of sulfur chemistry as well as for his textbook Chemistry of the Non-Metals, which appeared in several languages and many editions. Complementing his pioneering contributions to polysulfides, he authored many reviews on the subject. Steudel was born to a family of entrepreneurs in the Saxonian town of Kamenz. In 1954 he escaped to West Berlin, and started his university studies in chemistry in 1957 at the Free University Berlin under the supervision of Peter Wolfgang Schenk, whose research focused on sulfur monoxide and related chalcogen compounds. Steudel graduated in 1963. In 1965, he received his PhD in chemistry at Technische Universität Berlin (TU Berlin) where he subsequently made his habilitation work resulting in the venia legendi for inorganic chemistry in 1969. In the same year he was appointed professor of inorganic chemistr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |