|
Nitrosamine
Nitrosamines (or more formally ''N''-nitrosamines) are organic compounds produced by industrial processes. The chemical structure is , where R is usually an alkyl group. Nitrosamines have a nitroso group () that are "probable human carcinogens", bonded to a deprotonated amine. Most nitrosamines are carcinogenic in animals. A 2006 systematic review supports a "positive association between nitrite and nitrosamine intake and gastric cancer, between meat and processed meat intake and gastric cancer and oesophageal cancer, and between preserved fish, vegetable and smoked food intake and gastric cancer, but is not conclusive". Chemistry The organic chemistry of nitrosamines is well developed with regard to their syntheses, their structures, and their reactions. They usually are produced by the reaction of nitrous acid () and secondary amines, although other nitrosyl sources (e.g. , , Alkyl nitrite, RONO) have the same effect: : The nitrous acid usually arises from protonation of a nit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ranitidine
Ranitidine, previously sold under the brand name Zantac among others, is a medication used to decrease stomach acid production. It was commonly used in treatment of peptic ulcer disease, gastroesophageal reflux disease, and Zollinger–Ellison syndrome. It can be given by mouth, injection into a muscle, or injection into a vein. In September 2019, the probable carcinogen ''N''-nitrosodimethylamine (NDMA) was discovered in ranitidine products from a number of manufacturers, resulting in recalls. In April 2020, ranitidine was withdrawn from the United States market and suspended in the European Union and Australia due to these concerns. In 2022, these concerns were confirmed in a Taiwanese nationwide population study finding "significant trends of increased liver cancer risk with an increasing dose of ranitidine" (up to 22% higher than control) and increased gastric, pancreatic, lung and overall cancer risk. Common side effects include headaches, and pain or burning s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angiotensin II Receptor Blocker
Angiotensin II receptor blockers (ARBs), formally angiotensin II receptor type 1 (AT1) antagonists, also known as angiotensin receptor blockers, angiotensin II receptor antagonists, or AT1 receptor antagonists, are a group of pharmaceuticals that bind to and inhibit the angiotensin II receptor type 1 (AT1) and thereby block the arteriolar contraction and sodium retention effects of renin–angiotensin system. Their main uses are in the treatment of hypertension (high blood pressure), diabetic nephropathy ( kidney damage due to diabetes) and congestive heart failure. They ''selectively'' block the activation of the AT1 receptor, preventing the binding of angiotensin II compared to ACE inhibitors. ARBs and the similar-attributed ACE inhibitors are both indicated as the first-line antihypertensives in patients developing hypertension along with left-sided heart failure. However, ARBs appear to produce fewer adverse effects compared to ACE inhibitors. Medical uses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylnitrosamine
''N''-Nitrosodimethylamine (NDMA), also known as dimethylnitrosamine (DMN), is an organic compound with the formula (CH3)2NNO. It is one of the simplest members of a large class of nitrosamines. It is a volatile yellow oil. NDMA has attracted wide attention as being highly hepatotoxic and a known carcinogen in laboratory animals. Occurrence Drinking water Of more general concern, NDMA can be produced by water treatment by chlorination or chloramination. The question is the level at which it is produced. In the U.S. state of California, the allowable level is 10 nanograms/liter. The Canadian province of Ontario set the standard at 9 ng/L. The potential problem is greater for recycled water that can contain dimethylamine. Further, NDMA can form or be leached during treatment of water by anion exchange resins. Contamination of drinking water with NDMA is of particular concern due to the minute concentrations at which it is harmful and the difficulty in detecting it at these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Valsartan
Valsartan, sold under the brand name Diovan among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It belongs to a class of medications referred to as angiotensin II receptor blockers (ARBs). It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Common side effects include feeling tired, dizziness, high blood potassium, diarrhea, and joint pain. Other serious side effects may include kidney problems, low blood pressure, and angioedema. Use in pregnancy may harm the baby and use when breastfeeding is not recommended. It is an angiotensin II receptor antagonist and works by blocking the effects of angiotensin II. Valsartan was patented in 1990, and came into medical use in 1996. It is available as a generic medication. In 2022, it was the 117th most commonly prescribed medication in the United States, with more than 5million prescriptions. Medical uses Valsartan is used to treat high blood ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitroso
In organic chemistry, nitroso refers to a functional group in which the nitric oxide () group is attached to an organic moiety. As such, various nitroso groups can be categorized as ''C''-nitroso compounds (e.g., nitrosoalkanes; ), ''S''-nitroso compounds ( nitrosothiols; ), ''N''-nitroso compounds (e.g., nitrosamines, ), and ''O''-nitroso compounds ( alkyl nitrites; ). Synthesis Nitroso compounds can be prepared by the reduction of nitro compounds or by the oxidation of hydroxylamines. Ortho-nitrosophenols may be produced by the Baudisch reaction. In the Fischer–Hepp rearrangement, aromatic 4-nitrosoanilines are prepared from the corresponding nitrosamines. Properties Nitrosoarenes typically participate in a monomer–dimer equilibrium. The azobenzene ''N'',''N'-''dioxide (Ar(–O)N+=+N(O–)Ar) dimers, which are often pale yellow, are generally favored in the solid state, whereas the deep-green monomers are favored in dilute solution or at higher temperatures. They ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tobacco-specific Nitrosamines
Tobacco-specific nitrosamines (TSNAs) comprise one of the most important groups of carcinogens in tobacco products, particularly cigarettes (traditional and electronic) and fermented dipping snuff. Background These nitrosamine carcinogens are formed from nicotine and related compounds by a nitrosation reaction that occurs during the curing and processing of tobacco. Essentially the plant's natural alkaloids combine with nitrate forming the nitrosamines. They are called tobacco-specific nitrosamines because they are found only in tobacco products, and possibly in some other nicotine-containing products. The tobacco-specific nitrosamines are present in cigarette smoke and to a lesser degree in "smokeless" tobacco products such as dipping tobacco and chewing tobacco; additional information has shown that trace amounts of NNN and NNK have been detected in e-cigarettes. They are present in trace amounts in snus. They are important carcinogens in cigarette smoke, along with co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
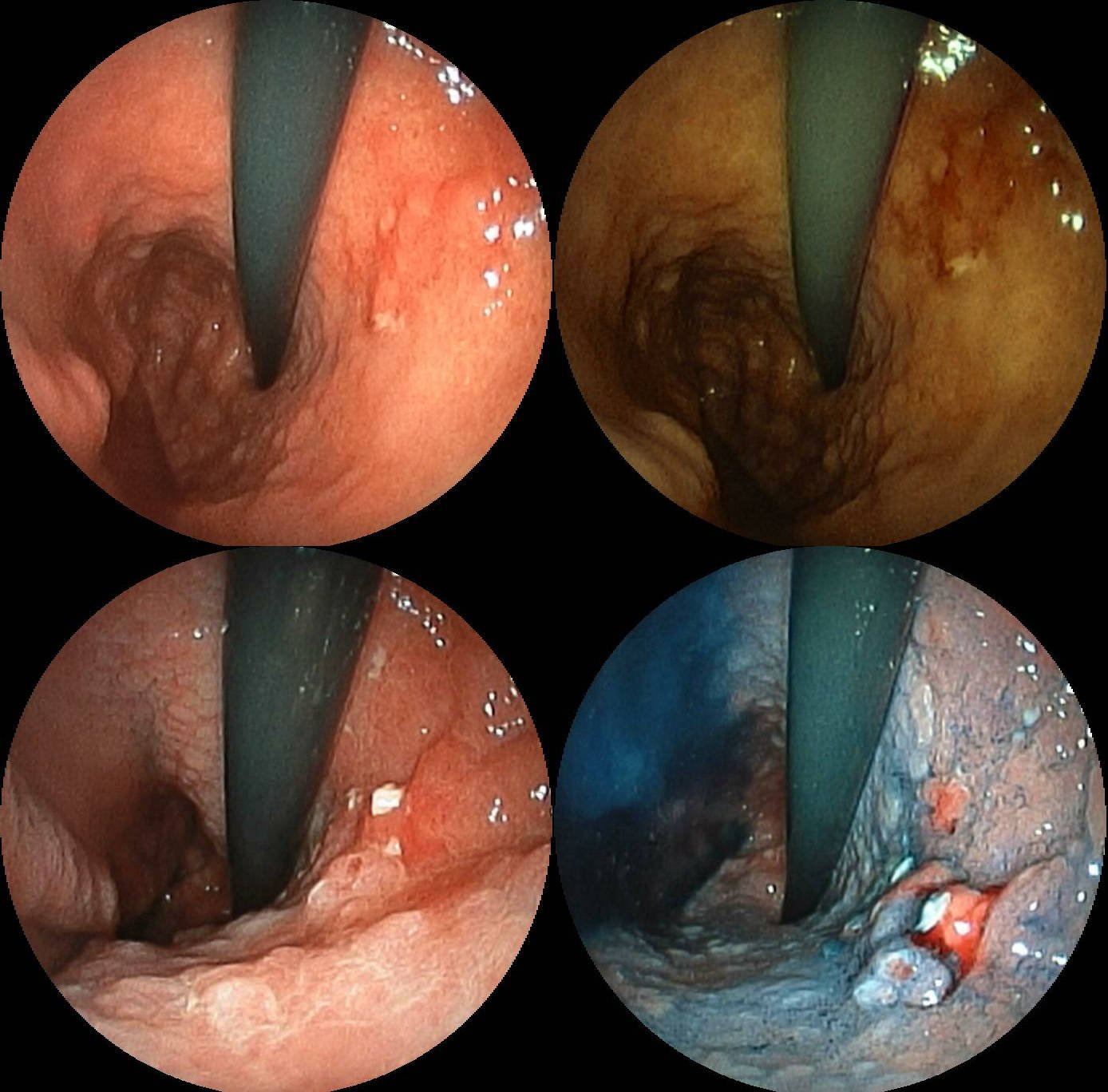
Gastric Cancer
Stomach cancer, also known as gastric cancer, is a malignant tumor of the stomach. It is a cancer that develops in the lining of the stomach. Most cases of stomach cancers are gastric carcinomas, which can be divided into a number of subtypes, including gastric adenocarcinomas. Lymphomas and mesenchymal tumors may also develop in the stomach. Early symptoms may include heartburn, upper abdominal pain, nausea, and loss of appetite. Later signs and symptoms may include weight loss, yellowing of the skin and whites of the eyes, vomiting, difficulty swallowing, and blood in the stool, among others. The cancer may spread from the stomach to other parts of the body, particularly the liver, lungs, bones, lining of the abdomen, and lymph nodes. The bacterium ''Helicobacter pylori'' accounts for more than 60% of cases of stomach cancer. Certain strains of ''H. pylori'' have greater risks than others. Smoking, dietary factors such as pickled vegetables and obesity are other ris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyldiazonium
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. The parent, compound where R is hydrogen, is diazenylium. Structure and general properties Arene derivatives According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group raises the ionization constant ''K''a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |