|
Nitinol
Nickel titanium, also known as nitinol, is a metal alloy of nickel and titanium, where the two elements are present in roughly equal atomic percentages. Different alloys are named according to the weight percentage of nickel; e.g., nitinol 55 and nitinol 60. Nitinol alloys exhibit two closely related and unique properties: the shape memory effect and superelasticity (also called pseudoelasticity). Shape memory is the ability of nitinol to undergo Deformation (engineering), deformation at one temperature, stay in its deformed shape when the external force is removed, then recover its original, undeformed shape upon heating above its "transformation temperature." Superelasticity is the ability for the metal to undergo large deformations and immediately return to its undeformed shape upon removal of the external load. Nitinol can undergo elastic deformations 10 to 30 times larger than alternative metals. Whether nitinol behaves with shape memory effect or superelasticity depends on w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shape Memory
In metallurgy, a shape-memory alloy (SMA) is an alloy that can be deformed when cold but returns to its pre-deformed ("remembered") shape when heated. It is also known in other names such as memory metal, memory alloy, smart metal, smart alloy, and muscle wire. The "memorized geometry" can be modified by fixating the desired geometry and subjecting it to a thermal treatment, for example a wire can be taught to memorize the shape of a coil spring. Parts made of shape-memory alloys can be lightweight, solid-state alternatives to conventional actuators such as hydraulic, pneumatic, and motor-based systems. They can also be used to make hermetic joints in metal tubing, and it can also replace a sensor-actuator closed loop to control water temperature by governing hot and cold water flow ratio. Overview The two most prevalent shape-memory alloys are copper-aluminium-nickel and nickel-titanium ( NiTi), but SMAs can also be created by alloying zinc, copper, gold and iron. Although ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitinol 60
NiTiNOL 60, or 60 NiTiNOL, is a Nickel Titanium alloy (nominally Ni-40wt% Ti) discovered in the late 1950s by the U. S. Naval Ordnance Laboratory (hence the "NOL" portion of the name NiTiNOL). Depending upon the heat treat history, 60 NiTiNOL has the ability to exhibit either superelastic properties in the hardened state or shape memory In metallurgy, a shape-memory alloy (SMA) is an alloy that can be deformed when cold but returns to its pre-deformed ("remembered") shape when heated. It is also known in other names such as memory metal, memory alloy, smart metal, smart alloy, ... characteristics in the softened state. Producing the material in any meaningful quantities, however, proved quite difficult by conventional methods and the material was largely forgotten. The composition and processing parameters have recently been revived by Summit Materials, LLC under the trademarked name SM-100. SM-100 maintains 60 NiTiNOL's combination of superb corrosion resistance [NASA t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superelasticity
In materials science, pseudoelasticity, sometimes called superelasticity, is an elastic (reversible) response to an applied stress, caused by a phase transformation between the austenitic and martensitic phases of a crystal. It is exhibited in shape-memory alloys. Overview Pseudoelasticity is from the reversible motion of domain boundaries during the phase transformation, rather than just bond stretching or the introduction of defects in the crystal lattice (thus it is not true super elasticity but rather pseudoelasticity). Even if the domain boundaries do become pinned, they may be reversed through heating. Thus, a pseudoelastic material may return to its previous shape (hence, ''shape memory'') after the removal of even relatively high applied strains. One special case of pseudoelasticity is called the Bain Correspondence. This involves the austenite/martensite phase transformation between a face-centered crystal lattice (FCC) and a body-centered tetragonal crystal st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naval Ordnance Laboratory
The Naval Ordnance Laboratory (NOL) was a facility in the White Oak, Maryland, White Oak area of Montgomery County, Maryland. The location is now used as the headquarters of the Food and Drug Administration, U.S. Food and Drug Administration. Origins The U.S. Navy Mine Unit, later the Mine Laboratory at the Washington, D.C., Navy Yard, was established in 1918, and the first Officer in Charge (OIC) arrived in February 1919, marking the beginning of the Laboratory. In 1929 the Mine Laboratory was merged with the Experimental Ammunition Station in Indian Head, Maryland, Indian Head to form the Naval Ordnance Laboratory. NOL began slowly, and it was not until the beginnings of World War II, when Germany's aircraft-laid magnetic mine began to cause serious problems for the Allies of World War II, Allies. As the importance of NOL's work became apparent, it also became apparent that there wasn't enough space at the Navy Yard to accommodate the necessary research facilities. In 1944, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deformation (engineering)
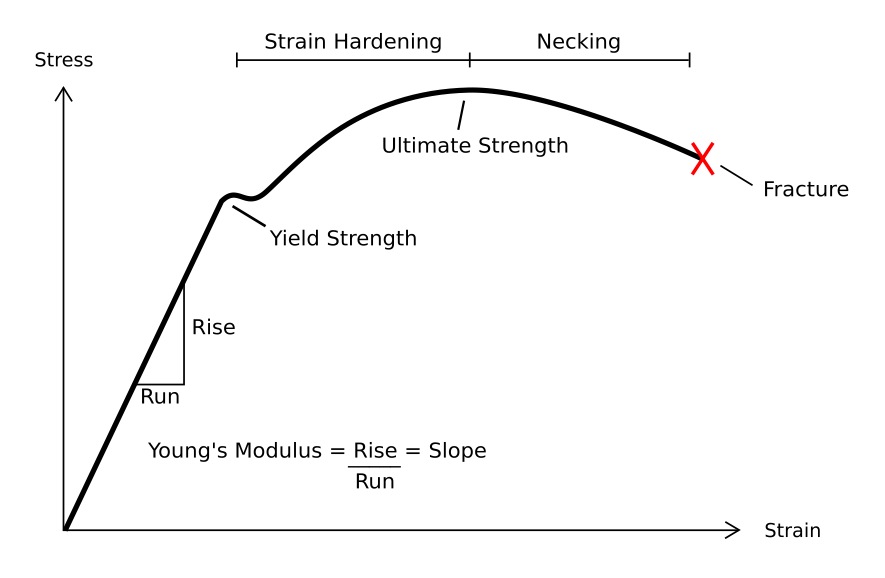
In engineering, deformation (the change in size or shape of an object) may be ''elastic'' or ''plastic''. If the deformation is negligible, the object is said to be ''rigid''. Main concepts Occurrence of deformation in engineering applications is based on the following background concepts: * ''Displacements'' are any change in position of a point on the object, including whole-body translations and rotations ( rigid transformations). * ''Deformation'' are changes in the relative position between internals points on the object, excluding rigid transformations, causing the body to change shape or size. * ''Strain'' is the ''relative'' ''internal'' deformation, the dimensionless change in shape of an infinitesimal cube of material relative to a reference configuration. Mechanical strains are caused by mechanical stress, ''see stress-strain curve''. The relationship between stress and strain is generally linear and reversible up until the yield point and the deformation is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martensitic Transformation
A diffusionless transformation, commonly known as displacive transformation, denotes solid-state alterations in crystal structures that do not hinge on the diffusion of atoms across extensive distances. Rather, these transformations manifest as a result of synchronized shifts in atomic positions, wherein atoms undergo displacements of distances smaller than the spacing between adjacent atoms, all while preserving their relative arrangement. An example of such a phenomenon is the martensitic transformation, a notable occurrence observed in the context of steel materials. The term "martensite" was originally coined to describe the rigid and finely dispersed constituent that emerges in steels subjected to rapid cooling. Subsequent investigations revealed that materials beyond ferrous alloys, such as non-ferrous alloys and ceramics, can also undergo diffusionless transformations. Consequently, the term "martensite" has evolved to encompass the resultant product arising from such tr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brass
Brass is an alloy of copper and zinc, in proportions which can be varied to achieve different colours and mechanical, electrical, acoustic and chemical properties, but copper typically has the larger proportion, generally copper and zinc. In use since prehistoric times, it is a substitutional alloy: atoms of the two constituents may replace each other within the same crystal structure. Brass is similar to bronze, a copper alloy that contains tin instead of zinc. Both bronze and brass may include small proportions of a range of other Chemical element, elements including arsenic, lead, phosphorus, aluminium, manganese and silicon. Historically, the distinction between the two alloys has been less consistent and clear, and increasingly museums use the more general term "list of copper alloys, copper alloy". Brass has long been a popular material for its bright gold-like appearance and is still used for drawer pulls and door handle, doorknobs. It has also been widely used to ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arne Ölander
Gustav Arne Ölander (31 December 1902 in Stockholm – 13 May 1984 in Stockholm) was a Swedish chemist, known for his discovery of the shape-memory effect in metal alloys. He was the son of Gustaf Ölander and Hilda Ölander née Norrman. Ölander became an associate professor of physical chemistry at Stockholm University in 1929. He was a professor of theoretical chemistry and electrochemistry at the Royal Institute of Technology 1936–1943, in inorganic and physical chemistry at Stockholm University 1943–1960, and in physical chemistry at Stockholm University 1960–1968. Arne Ölander became a member of the Academy of Engineering in 1943, was secretary of the Academy of Sciences Nobel Committees from 1943 to 1965, committee member of the International Union of Pure and Applied Chemistry The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |