|
Narrow Gap Semiconductors
Narrow-gap semiconductors are semiconducting materials with a magnitude of bandgap that is smaller than 0.7 eV, which corresponds to an infrared absorption cut-off wavelength over 2.5 micron. A more extended definition includes all semiconductors with bandgaps smaller than silicon (1.1 eV). Modern terahertz, infrared, and thermographic technologies are all based on this class of semiconductors. Narrow-gap materials made it possible to realize satellite remote sensing, photonic integrated circuits for telecommunications, and unmanned vehicle Li-Fi systems, in the regime of Infrared detector and thermography. They are also the materials basis for terahertz technology, including security surveillance of concealed weapon uncovering, safe medical and industrial imaging with terahertz tomography, as well as dielectric wakefield accelerators. Besides, thermophotovoltaics embedded with narrow-gap semiconductors can potentially use the traditionally wasted portion of solar energy that ta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconducting
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called " metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easily in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermophotovoltaics
Thermophotovoltaic (TPV) energy conversion is a direct conversion process from heat to electricity via photons. A basic thermophotovoltaic system consists of a hot object emitting thermal radiation and a photovoltaic cell similar to a solar cell but tuned to the spectrum being emitted from the hot object. As TPV systems generally work at lower temperatures than solar cells, their efficiencies tend to be low. Offsetting this through the use of multi-junction cells based on non-silicon materials is common, but generally very expensive. This currently limits TPV to niche roles like spacecraft power and waste heat collection from larger systems like steam turbines. General concept PV Typical photovoltaics work by creating a p–n junction near the front surface of a thin semiconductor material. When photons above the bandgap energy of the material hit atoms within the bulk lower layer, below the junction, an electron is photoexcited and becomes free of its atom. The junction create ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Arsenide
Indium arsenide, InAs, or indium monoarsenide, is a narrow-bandgap semiconductor composed of indium and arsenic. It has the appearance of grey cubic crystals with a melting point of 942 °C. Indium arsenide is similar in properties to gallium arsenide and is a direct bandgap material, with a bandgap of 0.35 eV at room temperature. Indium arsenide is used for the construction of infrared detectors, for the wavelength range of 1.0–3.8 μm. The detectors are usually photovoltaic photodiodes. Cryogenically cooled detectors have lower noise, but InAs detectors can be used in higher-power applications at room temperature as well. Indium arsenide is also used for making diode lasers. InAs is well known for its high electron mobility and narrow energy bandgap. It is widely used as a terahertz radiation Terahertz radiation – also known as submillimeter radiation, terahertz waves, tremendously high frequency (THF), T-rays, T-waves, T-light, T-lux or THz – consis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a permanent magnet itself. With the exception of extremely rare native iron deposits, it is the most magnetic of all the naturally occurring minerals on Earth. Naturally magnetized pieces of magnetite, called lodestone, will attract small pieces of iron, which is how ancient peoples first discovered the property of magnetism. Magnetite is black or brownish-black with a metallic luster, has a Mohs scale of mineral hardness, Mohs hardness of 5–6 and leaves a black streak (mineralogy), streak. Small grains of magnetite are very common in igneous rocks, igneous and metamorphic rocks. The chemical IUPAC name is iron(II,III) oxide and the common chemical name is ''ferrous-ferric oxide''. Properties In addition to igneous rocks, magnetite als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
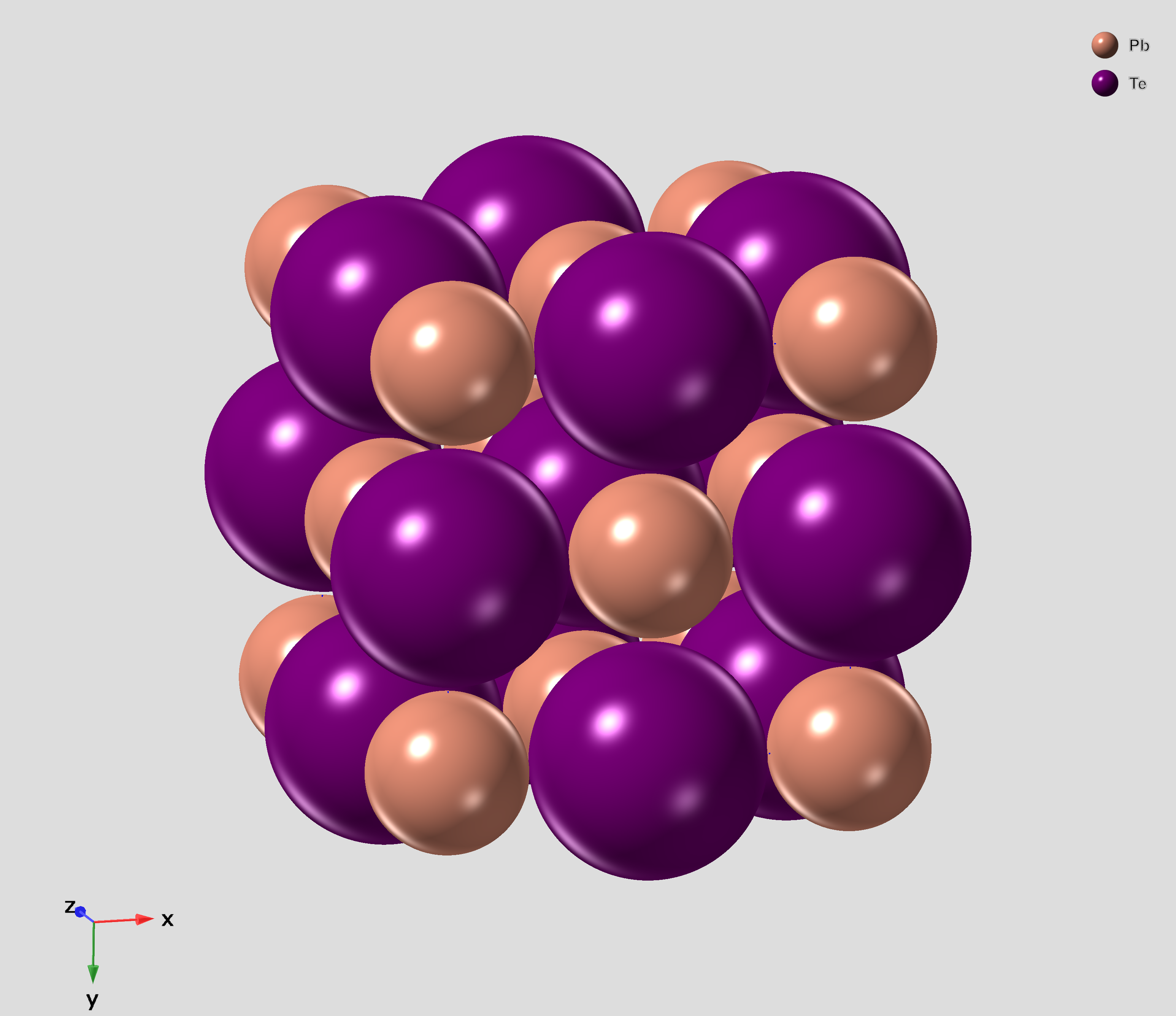
Lead Telluride
Lead telluride is a compound of lead (element), lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite. Properties * Dielectric constant ~1000. * Electron Effective mass (solid-state physics), Effective mass ~ 0.01Electron rest mass, ''m''e * Hole mobility, μp = 600 cm2 V−1 s−1 (0 K); 4000 cm2 V−1 s−1 (300 K) * Seebeck coefficient: ~326 μV/K (undoped, at 300K), ~200 μV/K (Ag-doped) Applications PbTe has proven to be a very important intermediate Thermoelectric materials, thermoelectric material. The performance of thermoelectric materials can be evaluated by the figure of merit, ZT=S^2\sigma T/\kappa, in which S is the Seebeck coefficient, \sigma is the Electrical resistivity and conductivity, electrical conducti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth. Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride minerals are the most notable natural gold compounds. However, they are not a commercially signif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(II) Sulfide
Lead(II) sulfide (also spelled '' sulphide'') is an inorganic compound with the formula Pb S. Galena is the principal ore and the most important compound of lead. It is a semiconducting material with niche uses. Formation, basic properties, related materials Addition of hydrogen sulfide or sulfide salts to a solution containing a lead salt, such as PbCl2, gives a black precipitate of lead sulfide. : Pb2+ + H2S → PbS↓ + 2 H+ This reaction is used in qualitative inorganic analysis. The presence of hydrogen sulfide or sulfide ions may be tested using "lead acetate paper." Like the related materials PbSe and PbTe, PbS is a semiconductor. In fact, lead sulfide was one of the earliest materials to be used as a semiconductor. Lead sulfide crystallizes in the sodium chloride motif, unlike many other IV-VI semiconductors. Since PbS is the main ore of lead, much effort has focused on its conversion. A major process involves smelting of PbS followed by reduction of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead Selenide
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. (Note that incorrectly identifies PbSe and other IV–VI semiconductors as indirect gap materials.) A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide. It may be formed by direct reaction between its constituent elements, lead and selenium. Infrared detection PbSe was one of the first materials found to be sensitive to the infrared radiation used for military applications. Early research works on the material as infrared detector were carried out during the 1930s and the first useful devices were processed by Germans, Americans and British during and just after World War II. Since then, PbSe has been commonly used as an infrared photodetector in multiple applications, from spectro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Zinc Telluride
Mercury zinc telluride (HgZnTe, MZT) is a telluride of mercury and zinc, an alloy of mercury telluride and zinc telluride. It is a narrow-gap semiconductor material. Mercury zinc telluride is used in infrared detectors and arrays for infrared imaging and infrared astronomy Infrared astronomy is a sub-discipline of astronomy which specializes in the astronomical observation, observation and analysis of astronomical objects using infrared (IR) radiation. The wavelength of infrared light ranges from 0.75 to 300 microm .... Mercury zinc telluride has better chemical, thermal, and mechanical stability than mercury cadmium telluride. The bandgap of MZT is more sensitive to composition fluctuations than that of MCT, which may be an issue for reproducible device fabrication. MZT is less amenable than MCT to fabrication of complex heterostructures by molecular beam epitaxy. See also * Cadmium zinc telluride * Mercury cadmium telluride External links National Pollutant Invent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury Cadmium Telluride
Hg1−''x''Cd''x''Te or mercury cadmium telluride (also cadmium mercury telluride, MCT, MerCad Telluride, MerCadTel, MerCaT or CMT) is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning the shortwave infrared to the very long wave infrared regions. The amount of cadmium (Cd) in the alloy can be chosen so as to tune the optical absorption of the material to the desired infrared wavelength. CdTe is a semiconductor with a bandgap of approximately at room temperature. HgTe is a semimetal, which means that its bandgap energy is zero. Mixing these two substances allows one to obtain any bandgap between 0 and 1.5 eV. Properties Physical Hg1−''x''Cd''x''Te has a zincblende structure with two interpenetrating face-centered cubic lattices offset by (1/4,1/4,1/4)''a''o in the primitive cell. The cations Cd and Hg are statistically mixed on the yellow sublattice while the Te anions form the grey sublattice in the imag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts) between the top of the valence band and the bottom of the conduction band in insulators and semiconductors. It is the energy required to promote an electron from the valence band to the conduction band. The resulting conduction-band electron (and the electron hole in the valence band) are free to move within the crystal lattice and serve as charge carriers to conduct electric current. It is closely related to the HOMO/LUMO gap in chemistry. If the valence band is completely full and the conduction band is completely empty, then electrons cannot move within the solid because there are no available states. If the electrons are not free to move within the crystal lattice, then there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group (periodic Table)
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the 14 f-block columns, between groups 2 and 3, are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because most chemical properties are dominated by the orbital location of the outermost electron. The modern numbering system of "group 1" to "group 18" has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since 1988. The 1-18 system is based on each atom's s, p and d electrons beyond those in atoms of the preceding noble gas. Two older incompatible naming schemes can assign the same number to different groups depending on the system being used. The older schemes were used by the Chemical Abstract Service (CAS, more popular in the United States), and by IUPAC be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |