|
Naloxol
Naloxol is an opioid antagonist closely related to naloxone. It exists in two isomeric forms, α-naloxol and β-naloxol. α-naloxol is a human metabolite of naloxone. Synthetically, α-naloxol can be prepared from naloxone by Organic redox reaction, reduction of the ketone group, and β-naloxol can be prepared from α-naloxol by a Mitsunobu reaction. Naloxol can be said to be the oxymorphol analogue of naloxone. See also * Naloxegol References 4,5-Epoxymorphinans Human drug metabolites Mu-opioid receptor antagonists Allyl compounds {{Analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naloxegol
Naloxegol ( INN; PEGylated naloxol; trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation. It was approved in 2014 in adult patients with chronic, non-cancer pain. Doses of 25 mg were found safe and well tolerated for 52 weeks. When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia. The most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting, and headache. Naloxegol was previously a Schedule II drug in the United States because of its chemical similarity to opium alkaloids. It was officially decontrolled in January 2015. It was reclassified as a prescription drug after the FDA and DEA concluded that the impermeability of the blood–brain barrier to this compound made it non-habit-forming, and so witho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
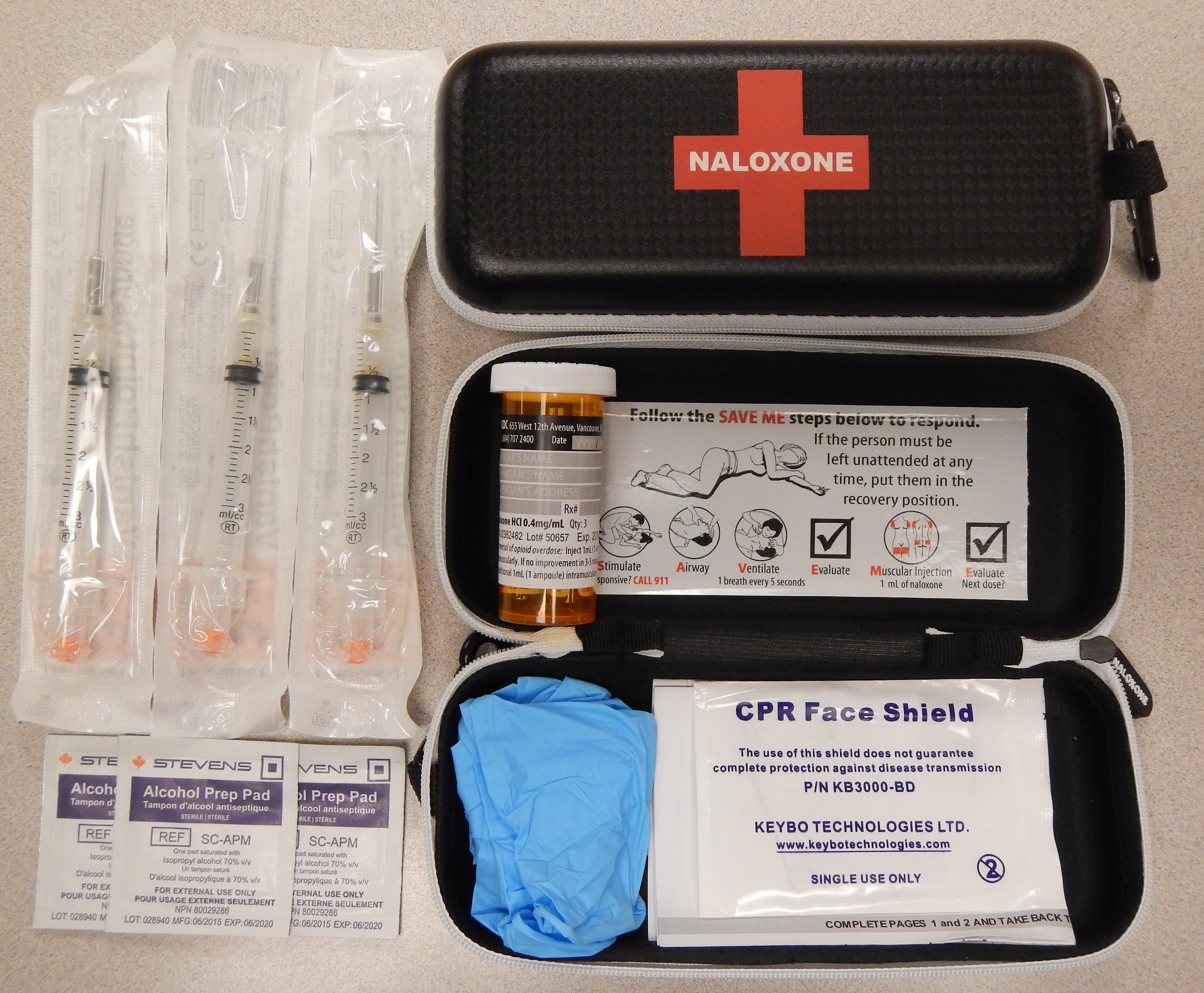
Naloxone
Naloxone, sold under the brand name Narcan among others, is an opioid antagonist, a medication used to reverse or reduce the effects of opioids. For example, it is used to restore breathing after an opioid overdose. Effects begin within two minutes when given intravenously, five minutes when injected into a muscle, and ten minutes as a nasal spray. Naloxone blocks the effects of opioids for 30 to 90 minutes. Administration to opioid-dependent individuals may cause symptoms of opioid withdrawal, including restlessness, agitation, nausea, vomiting, a fast heart rate, and sweating. To prevent this, small doses every few minutes can be given until the desired effect is reached. In those with previous heart disease or taking medications that negatively affect the heart, further heart problems have occurred. It appears to be safe in pregnancy, after having been given to a limited number of women. Naloxone is a non-selective and competitive opioid receptor antagonist. It revers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Antagonist
An opioid antagonist, or opioid receptor antagonist, is a receptor antagonist that acts on one or more of the opioid receptors. Naloxone and naltrexone are commonly used opioid antagonist drugs which are competitive antagonists that bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This effectively blocks the receptor, preventing the body from responding to opioids and endorphins. Some opioid antagonists are not pure antagonists but do produce some weak opioid partial agonist effects, and can produce analgesic effects when administered in high doses to opioid-naive individuals. Examples of such compounds include nalorphine and levallorphan. However, the analgesic effects from these specific drugs are limited and tend to be accompanied by dysphoria, most likely due to additional agonist action at the κ-opioid receptor. As they induce opioid withdrawal effects in people who are taking, or have recently used, opioid full agoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the existence or possibility of isomers. Isomers do not necessarily share similar chemical property, chemical or physical property, physical properties. Two main forms of isomerism are structural isomerism, structural (or constitutional) isomerism, in which ''chemical bond, bonds'' between the atoms differ; and stereoisomerism (or spatial isomerism), in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different Isotopologue, isotopologues. The depth of analy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism. The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, catalytic activity of their own (usually as a cofactor to an enzyme), defense, and interactions with other organisms (e.g. pigments, odorants, and pheromones). A primary metabolite is directly involved in normal "growth", development, and reproduction. Ethylene exemplifies a primary metabolite produced large-scale by industrial microbiology. A secondary metabolite is not directly involved in those processes, but usually has an important ecological function. Examples include antibiotics and pigments such as resins and terpenes etc. Some antibiotics use primary metabolites as precursors, such as actinomycin, which is created from the primary metabolite tryptophan. Some sugars are metabolites, such as fructose or glucose, which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidations electrons are r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' are methyl), with the formula . Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, ''e.g.'', testosterone, and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered retained IUPAC names, although some introdu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitsunobu Reaction
The Mitsunobu reaction is an organic reaction that converts an alcohol into a variety of functional groups, such as an ester, using triphenylphosphine and an azodicarboxylate such as diethyl azodicarboxylate (DEAD) or diisopropyl azodicarboxylate (DIAD). Although DEAD and DIAD are most commonly used, there are a variety of other azodicarboxylates available which facilitate an easier workup and/or purification and in some cases, facilitate the use of more basic nucleophiles. It was discovered by Oyo Mitsunobu (1934–2003). In a typical protocol, one dissolves the alcohol, the carboxylic acid, and triphenylphosphine in tetrahydrofuran or other suitable solvent (e.g. diethyl ether), cool to 0 °C using an ice-bath, slowly add the DEAD dissolved in THF, then stir at room temperature for several hours. The alcohol reacts with the phosphine to create a good leaving group then undergoes an inversion of stereochemistry in classic SN2 fashion as the nucleophile displaces it. A c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxymorphol
Oxymorphol is oxymorphone which has been hydrogenated at the 6-position and consists of a mixture of 4,5α-Epoxy-17-methylmorphinan-3,6β,14-triol and 4,5α-Epoxy-17-methylmorphinan-3,6α,14-triol (hydromorphinol). It is produced by the human body as an active metabolite of oxymorphone and some bacteria as an intermediate in turning morphine into hydromorphone. It can also be manufactured and is the subject of patents by drug companies looking for new semi-synthetic analgesics and cough suppressants. A derivative of oxymorphol, 8-hydroxy-6-α-oxymorphol, was discovered in the first decade of the 21st century and the subject of a patent application by Endo Pharmaceuticals for an analgesic and antitussive. References {{Opioidergics 4,5-Epoxymorphinans Opioid metabolites Semisynthetic opioids Mu-opioid receptor agonists ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Drug Metabolites
Humans (''Homo sapiens'') or modern humans are the most common and widespread species of primate, and the last surviving species of the genus ''Homo''. They are great apes characterized by their hairlessness, bipedalism, and high intelligence. Humans have large brains, enabling more advanced cognitive skills that facilitate successful adaptation to varied environments, development of sophisticated tools, and formation of complex social structures and civilizations. Humans are highly social, with individual humans tending to belong to a multi-layered network of distinct social groups — from families and peer groups to corporations and political states. As such, social interactions between humans have established a wide variety of values, social norms, languages, and traditions (collectively termed institutions), each of which bolsters human society. Humans are also highly curious: the desire to understand and influence phenomena has motivated humanity's developmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |