|
Monoterpene
Monoterpenes are a class of terpenes that consist of two isoprene units and have the molecular formula C10H16. Monoterpenes may be linear (acyclic) or contain rings (monocyclic and bicyclic). Modified terpenes, such as those containing oxygen functionality or missing a methyl group, are called monoterpenoids. Monoterpenes and monoterpenoids are diverse. They have relevance to the pharmaceutical, cosmetic, agricultural, and food industries. Biosynthesis Monoterpenes are derived biosynthetically from units of isopentenyl pyrophosphate, which is formed from acetyl-CoA via the intermediacy of mevalonic acid in the HMG-CoA reductase pathway. An alternative, unrelated biosynthesis pathway of IPP is known in some bacterial groups and the plastids of plants, the so-called MEP-(2-methyl-D-erythritol-4-phosphate) pathway, which is initiated from C5 sugars. In both pathways, IPP is isomerized to DMAPP by the enzyme isopentenyl pyrophosphate isomerase. Geranyl pyrophosphate is the precu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly Pinophyta, conifers. In plants, terpenes and terpenoids are important mediators of ecological biological interaction, interactions, while some insects use some terpenes as a form of defense. Other functions of terpenoids include cell growth modulation and plant elongation, light harvesting and photoprotection, and membrane permeability and fluidity control. Terpenes are classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine. The one terpene that has major applications is natural rubber (i.e., polyisoprene). The possibility that other terpenes could be used as precursors to pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Halomon
Halomon is a polyhalogenated monoterpene first isolated from the marine red algae '' Portieria hornemannii''. Halomon has attracted research interest because of its promising profile of selective cytotoxicity that suggests its potential use as an antitumor agent. Halomon is in a class of chemical compounds known as halocarbons, which are often potent alkylating agents which may be toxic to individual cells or to living organisms. The red algae that naturally produce halomon and other related compounds probably do so as a poisonous defense against fish or other marine life that may see it as a potential source of food. Halomon, however, is a selective toxin; studies at the National Cancer Institute have indicated that it is more toxic to certain types of tumor cells than to other cells. The algae that produces halomon is difficult to locate, identify, and collect and the concentration of halomon in the organism is extremely low. Therefore, obtaining a sufficient amount of hal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
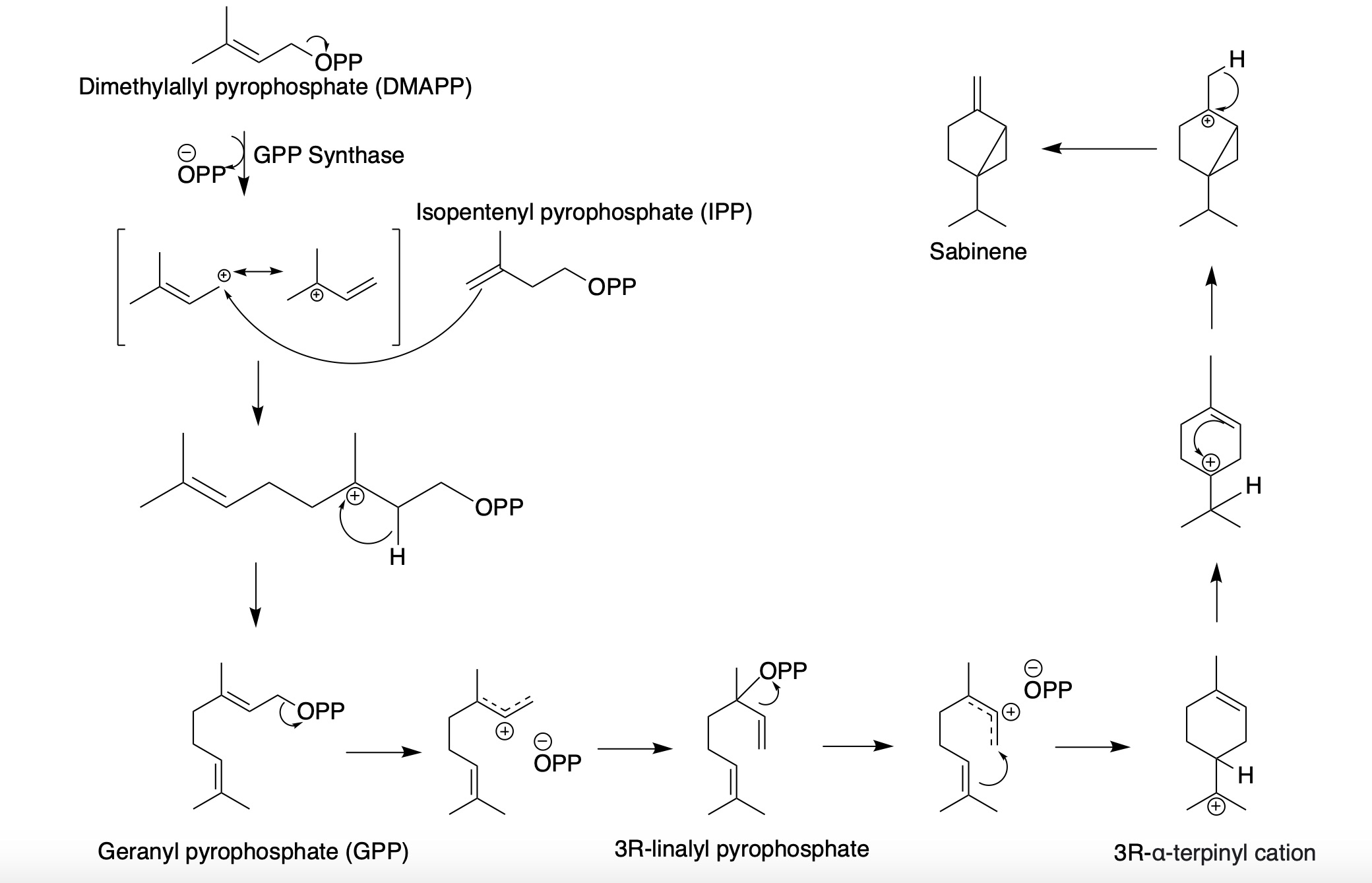
Sabinene
Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (''Quercus ilex'') and Norway spruce (''Picea abies''). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring. Sabinene is one of the chemical compounds that contributes to the spiciness of black pepper and is a major constituent of carrot seed oil. It also occurs in tea tree oil at a low concentration. It is also present in the essential oil obtained from nutmeg, '' Laurus nobilis'', and '' Clausena anisata''. Biosynthesis Sabinene, a bicyclic monoterpene, is present in the (+) and (-) enantiomer In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...s. It is biosynthesized from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Myrcene
Myrcene, or β-myrcene, is a terpene, monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from ''Myrcia'', from which it gets its name. It is an intermediate in the production of several fragrances. A less-common isomeric form, having one of the three alkene units in a different position, is α-myrcene. Production Myrcene is often produced commercially by the pyrolysis (400 °C) of β-pinene, which is obtained from turpentine. It is rarely obtained directly from plants. Plants biosynthesize myrcene via geranyl pyrophosphate (GPP), which isomerizes into linalool, linalyl pyrophosphate. An elimination reaction, releasing the pyrophosphate (OPP) and a proton, completes the conversion. Occurrence It could in principle be extracted from any number of plants, such as verbena or wild thyme, the leaves of which contain up to 40% by weight of myrcene. Many other plants contain myrcene, sometimes in substantial amounts. Some of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carvone
Carvone is a member of a family of chemicals called terpenoids. Carvone is found naturally in many essential oils, but is most abundant in the oils from seeds of caraway (''Carum carvi''), spearmint (''Mentha spicata''), and dill. Uses Food applications Both carvones are used in the food and flavor industry. As the compound most responsible for the flavor of caraway, dill, and spearmint, carvone has been used for millennia in food. Food applications are mainly met by carvone made from limonene. ''R''-(−)-Carvone is also used for air freshening products and, like many essential oils, oils containing carvones are used in aromatherapy and alternative medicine. Agriculture ''S''-(+)-Carvone is also used to prevent premature sprouting of potatoes during storage, being marketed in the Netherlands for this purpose under the name ''Talent''. Insect control ''R''-(−)-Carvone has been approved by the U.S. Environmental Protection Agency for use as a mosquito repellent. Stereoiso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Limonene
Limonene () is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, is a flavoring agent in food manufacturing. It is also used in chemical synthesis as a precursor to carvone and as a renewables-based solvent in cleaning products. The less common (−)-isomer has a piny, turpentine-like odor, and is found in the edible parts of such plants as caraway, dill, and bergamot orange plants. Limonene takes its name from Italian ''limone'' ("lemon"). Limonene is a chiral molecule, and biological sources produce one enantiomer: the principal industrial source, citrus fruit, contains (+)-limonene (''d''-limonene), which is the (''R'')-enantiomer. (+)-Limonene is obtained commercially from citrus fruits through two primary methods: centrifugal separation or steam distillation. In plants (+)-Limonene ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Citral
Citral is an acyclic monoterpene aldehyde. Being a monoterpene, it is made of two isoprene units. Citral is a collective term which covers two geometric isomers that have their own separate names; the ''E''-isomer is named geranial (''trans''-citral; α-citral) or citral A. The ''Z''-isomer is named neral (''cis''-citral; β-citral) or citral B. These stereoisomers occur as a mixture, often not in equal proportions; e.g. in essential oil of Australian ginger, the neral to geranial ratio is 0.61. Natural Occurrence Citral is present in the volatile oils of several plants: Further, in the lipid fraction (essential oil) of Australian ginger (51–71%) Of the many sources of citral, the Australian myrtaceous tree, lemon myrtle, '' Backhousia citriodora'' F. Muell. (of the family Myrtaceae), is considered superior. Uses Citral is a precursor in the industrial production of vitamin A, vitamin E, vitamin K. Citral is also precursor to lycopene, ionone and methylionone. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Myrcene
Myrcene, or β-myrcene, is a terpene, monoterpene. A colorless oil, it occurs widely in essential oils. It is produced mainly semi-synthetically from ''Myrcia'', from which it gets its name. It is an intermediate in the production of several fragrances. A less-common isomeric form, having one of the three alkene units in a different position, is α-myrcene. Production Myrcene is often produced commercially by the pyrolysis (400 °C) of β-pinene, which is obtained from turpentine. It is rarely obtained directly from plants. Plants biosynthesize myrcene via geranyl pyrophosphate (GPP), which isomerizes into linalool, linalyl pyrophosphate. An elimination reaction, releasing the pyrophosphate (OPP) and a proton, completes the conversion. Occurrence It could in principle be extracted from any number of plants, such as verbena or wild thyme, the leaves of which contain up to 40% by weight of myrcene. Many other plants contain myrcene, sometimes in substantial amounts. Some of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ocimene
Ocimenes are a group of isomeric hydrocarbons. The ocimenes are monoterpenes found within a variety of plants and fruits. α-Ocimene and the two β-ocimenes differ in the position of the isolated double bond: it is terminal in the alpha isomer. α-Ocimene is ''cis-''3,7-dimethyl-1,3,7-octatriene. β-Ocimene is ''trans-''3,7-dimethyl-1,3,6-octatriene. β-Ocimene exists in two stereoisomeric forms, ''cis'' and ''trans,'' with respect to the central double bond. The ocimenes are often found naturally as mixtures of the various forms. The mixture, as well as the pure compounds, are oils with a pleasant odor. They are used in perfumery for their sweet herbal scent and are believed to act as plant defense and have anti-fungal properties. Like the related acyclic terpene myrcene, ocimenes are unstable in air.Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe, Horst Surburg "Flavors and Fragrance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Thujene
Thujene (or α-thujene) is a natural organic compound classified as a monoterpene. It is found in the essential oils of a variety of plants, and contributes pungency to the flavor of some herbs such as Summer savory.''PDR for Herbal Medicines'', Third Edition, Joerg Gruenwald (Editor), page 802. The term ''thujene'' usually refers to α-thujene. A less common chemically related double-bond isomer is known as β-thujene (or 2-thujene). Another double-bond isomer is known as sabinene. {, class="toccolours" border="1" style="margin: 0 0 1em 1em; border-collapse: collapse;" , colspan="3" align="center" , Chemical structure comparison , - , , , , - , align="center", α-Thujene , align="center", β-Thujene , align="center", Sabinene See also * Thujone * Umbellulone, a thujene derivative References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Camphene
Camphene is a bicyclic organic compound. It is one of the most pervasive monoterpenes. As with other terpenes, it is insoluble in water, flammable, colorless, and has a pungent smell. It is a minor constituent of many essential oils such as turpentine, cypress oil, camphor oil, citronella oil, neroli, ginger oil, valerian, and mango. It is produced industrially by isomerization of the more common alpha-pinene using a solid acid catalyst such as titanium dioxide. Camphene is used in the preparation of fragrances and as a food additive for flavoring. These include isobornyl acetate. Biosynthesis Camphene is biosynthesized from linalyl pyrophosphate via a sequence of carbocation Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...ic intermediates. : References {{Authority ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isoprene
Isoprene, or 2-methyl-1,3-butadiene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form it is a colorless volatile liquid. It is produced by many plants and animals (including humans) and its polymers are the main component of natural rubber. History and etymology Charles Greville Williams, C. G. Williams named the compound in 1860 after obtaining it from the pyrolysis of natural rubber. He correctly deduced the mass shares of carbon and hydrogen (but arrived at an incorrect formula C10H8 because the modern atomic weight of carbon was not adopted until the Karlsruhe Congress held later that year). He did not specify the reasons for the name, but it is hypothesized that it came from "propylene" with which isoprene shares some physical and chemical properties. The first one to observe recombination of isoprene into rubber-like substance was in 1879, and William A. Tilden identified its structure five years later. Natural occurrences Is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |