|
Modified-release
Modified-release dosage is a mechanism that (in contrast to immediate-release dosage) delivers a drug with a delay after its administration (delayed-release dosage) or for a prolonged period of time (extended-release R, XR, XLdosage) or to a specific target in the body (targeted-release dosage).Pharmaceutics: Drug Delivery and Targeting p. 7-13 Sustained-release dosage forms are s designed to release (liberate) a drug at a predetermined rate in order to maintain a constant drug concentrat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concerta 54mg OROS
Methylphenidate, sold under the brand names Ritalin ( ) and Concerta ( ) among others, is a central nervous system (CNS) stimulant used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy. It may be taken Oral administration, by mouth or applied to the skin, and different formulations have varying durations of effect. For ADHD, the effectiveness of methylphenidate is comparable to atomoxetine but modestly lower than amphetamines, alleviating the Executive functions, executive functioning deficits of sustained attention, inhibition, working memory, reaction time and emotional self-regulation. Common adverse reactions of methylphenidate include euphoria, Mydriasis, dilated pupils, tachycardia, palpitations, headache, insomnia, anxiety, hyperhidrosis, weight loss, Anorexia (symptom), decreased appetite, Xerostomia, dry mouth, nausea, and abdominal pain. Drug withdrawal, Withdrawal symptoms may include chills, Depression (mood), depression, drowsiness ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micro-encapsulation
Microencapsulation is a process in which tiny particles or droplets are surrounded by a coating to give small capsules, with useful properties. In general, it is used to incorporate food ingredients, enzymes, cells or other materials on a micro metric scale. Microencapsulation can also be used to enclose solids, liquids, or gases inside a micrometric wall made of hard or soft soluble film, in order to reduce dosing frequency and prevent the degradation of pharmaceuticals. In its simplest form, a microcapsule is a small sphere comprising a near-uniform wall enclosing some material. The enclosed material in the microcapsule is referred to as the core, internal phase, or fill, whereas the wall is sometimes called a shell, coating, or membrane. Some materials like lipids and polymers, such as alginate, may be used as a mixture to trap the material of interest inside. Most microcapsules have pores with diameters between a few nanometers and a few micrometers. Materials generally us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dose (biochemistry)
A dose is a measured quantity of a medicine, nutrient, or pathogen that is delivered as a unit. The greater the quantity delivered, the larger the dose. Doses are most commonly measured for compounds in medicine. The term is usually applied to the quantity of a drug or other agent administered for therapeutic purposes, but may be used to describe any case where a substance is introduced to the body. In nutrition, the term is usually applied to how much of a specific nutrient is in a person's diet or in a particular food, meal, or dietary supplement. For bacterial or Virus, viral agents, dose typically refers to the amount of the pathogen required to infect a host. In clinical pharmacology, ''dose'' refers to the amount of drug administered to a person, and Dosage (pharmacology), ''dosage'' is a fuller description that includes not only the dose (e.g., "500 mg") but also the frequency and duration of the treatment (e.g., "twice a day for one week"). Exposure assessment, '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dosage Form
Dosage forms (also called unit doses) are pharmaceutical drug products presented in a specific form for use. They contain a mixture of active ingredients and inactive components ( excipients), configured in a particular way (such as a capsule shell) and apportioned into a specific dose. For example, two products may both be amoxicillin, but one may come in 500 mg capsules, while another may be in 250 mg chewable tablets. The term unit dose can also refer to non-reusable packaging, particularly when each drug product is individually packaged. However, the FDA differentiates this by referring to it as ''unit-dose "packaging" or "dispensing"''. Depending on the context, ''multi(ple) unit dose'' may refer to multiple distinct drug products ''packaged'' together or a ''single'' product containing multiple drugs and/or doses. Formulations The term dosage form may also sometimes refer only to the pharmaceutical formulation of a drug product's constituent substances, witho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
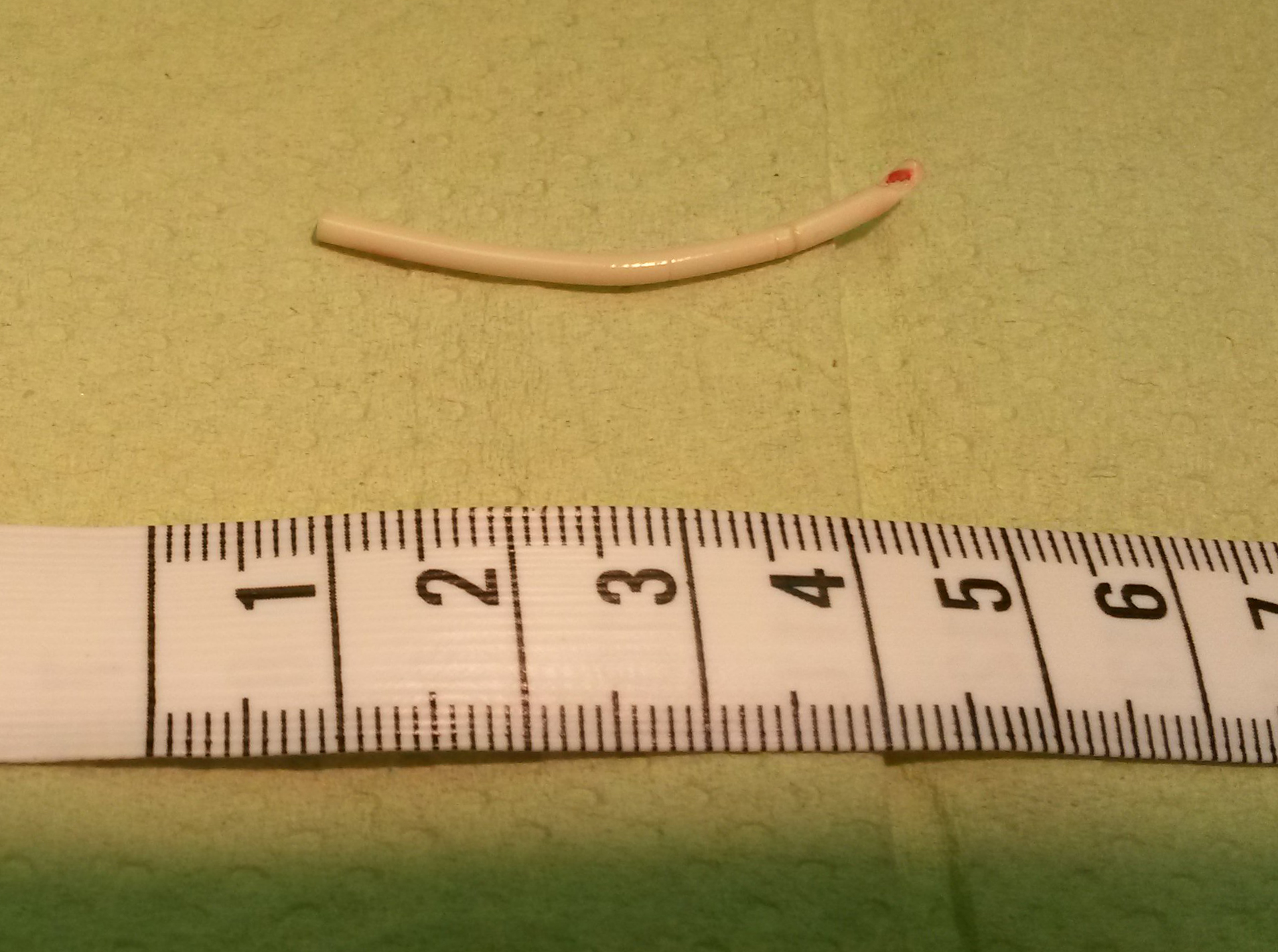
Contraceptive Implant
A contraceptive implant is an implantable medical device used for the purpose of birth control. The implant may depend on the timed release of hormones to hinder ovulation or sperm development, the ability of copper to act as a natural spermicide within the uterus, or it may work using a non-hormonal, physical blocking mechanism. As with other contraceptives, a contraceptive implant is designed to prevent pregnancy, but it does not protect against sexually transmitted infections. Women Implant The contraceptive implant is hormone-based and highly effective, approved in more than 60 countries and used by millions of women around the world. The typical implant is a small flexible tube measuring about in length. It is most commonly inserted subdermally in the inner portion of the upper, non-dominant arm by a trained and certified health care provider. After insertion, it prevents pregnancy by releasing progestin which inhibits ovulation. The two most common versions are the singl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dose Dumping
Dose dumping is a phenomenon of drug metabolism in which environmental factors can cause the premature and exaggerated release of a drug. This can greatly increase the concentration of a drug in the body and thereby produce adverse effects or even drug-induced toxicity. Dose dumping is most commonly seen in drugs taken by mouth and digested in the gastrointestinal tract. Around the same time patients take their medication, they can also ingest other substances like fatty meals or alcohol that increase drug delivery. The substances may act on the drug's capsule to speed up drug release, or they may stimulate the body's absorptive surfaces to increase the rate of drug uptake. Dose dumping is a disadvantage found in extended release dosage form. In general, drug companies try to avoid drugs with significant dose dumping effects. Such drugs are prone to problems and are often pulled from the market. Such was the case with the pain medication Palladone Once Daily formulation due t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Therapeutic Index
The therapeutic index (TI; also referred to as therapeutic ratio) is a quantitative measurement of the relative safety of a drug with regard to risk of overdose. It is a comparison of the amount of a therapeutic agent that causes toxicity to the amount that causes the therapeutic effect. The related terms therapeutic window or safety window refer to a range of doses optimized between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity. Classically, for clinical indications of an approved drug, TI refers to the ratio of the dose of the drug that causes adverse effects at an incidence/severity not compatible with the targeted indication (e.g. toxic dose in 50% of subjects, TD) to the dose that leads to the desired pharmacological effect (e.g. efficacious dose in 50% of subjects, ED). In contrast, in a drug development setting TI is calculated based on plasma exposure levels. In the early days of pharmaceu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Half-life
Biological half-life (elimination half-life, pharmacological half-life) is the time taken for concentration of a drug, biological substance (such as a medication) to decrease from its maximum concentration (chemistry), concentration (Cmax (pharmacology), Cmax) to half of Cmax in the blood plasma. It is denoted by the abbreviation t_. This is used to measure the removal of things such as metabolites, drugs, and signalling molecules from the body. Typically, the biological half-life refers to the body's natural detoxification (cleansing) through liver metabolism and through the excretion of the measured substance through the kidneys and intestines. This concept is used when the rate of removal is roughly Exponential function, exponential. In a medical context, half-life explicitly describes the time it takes for the blood plasma concentration of a substance to halve (''plasma half-life'') its steady-state when circulating in the full blood of an organism. This measurement is usefu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Active Transport
In cellular biology, active transport is the movement of molecules or ions across a cell membrane from a region of lower concentration to a region of higher concentration—against the concentration gradient. Active transport requires cellular energy to achieve this movement. There are two types of active transport: primary active transport that uses adenosine triphosphate (ATP), and secondary active transport that uses an electrochemical gradient. This process is in contrast to passive transport, which allows molecules or ions to move down their concentration gradient, from an area of high concentration to an area of low concentration, with energy. Active transport is essential for various physiological processes, such as nutrient uptake, hormone secretion, and nig impulse transmission. For example, the sodium-potassium pump uses ATP to pump sodium ions out of the cell and potassium ions into the cell, maintaining a concentration gradient essential for cellular function. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capsule (pharmacy)
In the manufacture of pharmaceuticals, encapsulation refers to a range of dosage forms—techniques used to enclose medicines—in a relatively stable shell known as a capsule, allowing them to, for example, be taken orally or be used as suppositories. The two main types of capsules are: * Hard-shelled capsules, which contain dry, powdered ingredients or miniature pellets made by ''e.g.'' processes of extrusion or spheronization. These are made in two-halves: a smaller-diameter "body" that is filled and then sealed using a larger-diameter "cap". * Soft-shelled capsules, primarily used for oils and for active ingredients that are dissolved or suspended in oil. Both of these classes of capsules are made from aqueous solutions of gelling agents, such as animal protein (mainly gelatin) or plant polysaccharides or their derivatives (such as carrageenans and modified forms of starch and cellulose). Other ingredients can be added to the gelling agent solution including plasticizers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Route Of Administration
In pharmacology and toxicology, a route of administration is the way by which a medication, drug, fluid, poison, or other substance is taken into the body. Routes of administration are generally classified by the location at which the substance is applied. Common examples include oral administration, oral and intravenous therapy, intravenous administration. Routes can also be classified based on where the target of action is. Action may be topical medication, topical (local), enteral administration, enteral (system-wide effect, but delivered through the gastrointestinal tract), or #Parenteral, parenteral (systemic action, but is delivered by routes other than the GI tract). Route of administration and dosage form are aspects of drug delivery. Classification Routes of administration are usually classified by application location (or exposition). The route or course the active substance takes from application location to the location where it has its target effect is usually rat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patented
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an enabling disclosure of the invention."A patent is not the grant of a right to make or use or sell. It does not, directly or indirectly, imply any such right. It grants only the right to exclude others. The supposition that a right to make is created by the patent grant is obviously inconsistent with the established distinctions between generic and specific patents, and with the well-known fact that a very considerable portion of the patents granted are in a field covered by a former relatively generic or basic patent, are tributary to such earlier patent, and cannot be practiced unless by license thereunder." – ''Herman v. Youngstown Car Mfg. Co.'', 191 F. 579, 584–85, 112 CCA 185 (6th Cir. 1911) In most countries, patent rights fall under private law and the patent holder mu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |