|
Mir-210 MicroRNA
In molecular biology mir-210 microRNA is a short non-coding RNA, RNA molecule. MicroRNAs function to regulate the expression levels of other genes by several mechanisms. mir-210 has been strongly linked with the Hypoxia (medical), hypoxia pathway, and is upregulated in response to Hypoxia-inducible factors. It is also overexpressed in cells affected by cardiac disease and tumours. MiRNA-210 in particular, has been studied for its effects in rescuing cardiac function after myocardial infarcts via the up-regulation of angiogenesis and inhibition of cardiomyocyte apoptosis. Myocardial infarction therapy Myocardial infarction is cardiac tissue necrosis that results from occlusion of blood supply via coronary arteries, thereby starving cells of oxygen and nutrients (termed ischemia). Prolong ischemia will eventually kill the cells and the destruction of cardiac cells leads to tissue death, which can lead to heart failure. Delivery of miRNA-210 to an ischemic heart improves heart func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MicroRNA
MicroRNA (miRNA) are small, single-stranded, non-coding RNA molecules containing 21 to 23 nucleotides. Found in plants, animals and some viruses, miRNAs are involved in RNA silencing and post-transcriptional regulation of gene expression. miRNAs base-pair to complementary sequences in mRNA molecules, then gene silence said mRNA molecules by one or more of the following processes: (1) cleavage of mRNA strand into two pieces, (2) destabilization of mRNA by shortening its poly(A) tail, or (3) translation of mRNA into proteins. This last method of gene silencing is the least efficient of the three, and requires the aid of ribosomes. miRNAs resemble the small interfering RNAs (siRNAs) of the RNA interference (RNAi) pathway, except miRNAs derive from regions of RNA transcripts that fold back on themselves to form short hairpins, whereas siRNAs derive from longer regions of double-stranded RNA. The human genome may encode over 1900 miRNAs, although more recent analysis s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
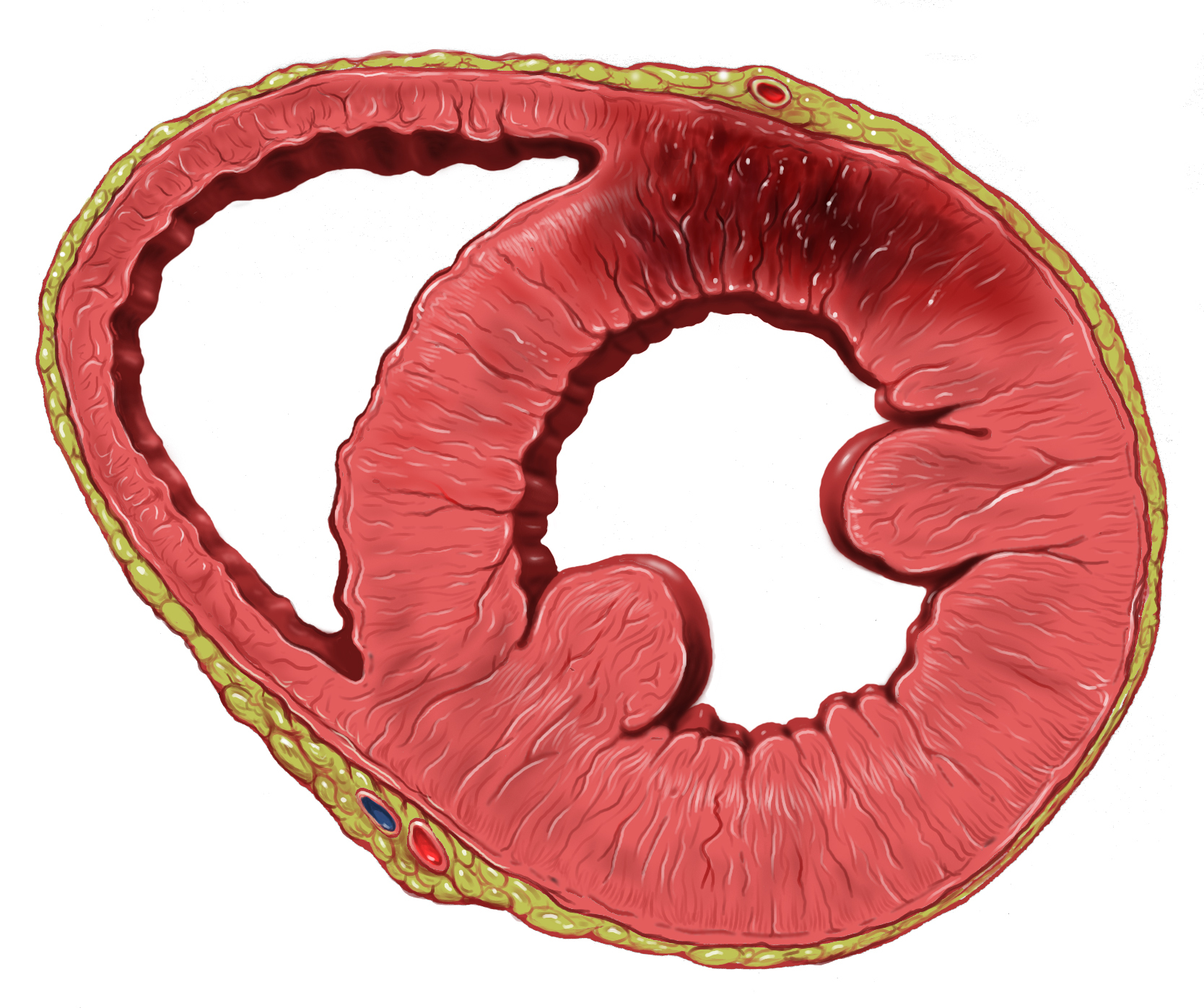
Myocardial Infarction
A myocardial infarction (MI), commonly known as a heart attack, occurs when blood flow decreases or stops to the coronary artery of the heart, causing damage to the heart muscle. The most common symptom is chest pain or discomfort which may travel into the shoulder, arm, back, neck or jaw. Often it occurs in the center or left side of the chest and lasts for more than a few minutes. The discomfort may occasionally feel like heartburn. Other symptoms may include shortness of breath, nausea, feeling faint, a cold sweat or feeling tired. About 30% of people have atypical symptoms. Women more often present without chest pain and instead have neck pain, arm pain or feel tired. Among those over 75 years old, about 5% have had an MI with little or no history of symptoms. An MI may cause heart failure, an irregular heartbeat, cardiogenic shock or cardiac arrest. Most MIs occur due to coronary artery disease. Risk factors include high blood pressure, smoking, diabetes, l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase-8
Caspase-8 is a caspase protein, encoded by the ''CASP8'' gene. It most likely acts upon caspase-3. ''CASP8'' orthologs have been identified in numerous mammals for which complete genome data are available. These unique orthologs are also present in birds. Function The ''CASP8'' gene encodes a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes composed of a prodomain, a large protease subunit, and a small protease subunit. Activation of caspases requires proteolytic processing at conserved internal aspartic residues to generate a heterodimeric enzyme consisting of the large and small subunits. This protein is involved in the programmed cell death induced by Fas and various apoptotic stimuli. The N-terminal FADD-like death effector domain of this protein suggests that it may interact with Fas-interacting protein FADD. This protei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caspase-3
Caspase-3 is a caspase protein that interacts with caspase-8 and caspase-9. It is encoded by the ''CASP3'' gene. ''CASP3'' orthologs have been identified in numerous mammals for which complete genome data are available. Unique orthologs are also present in birds, lizards, lissamphibians, and teleosts. The CASP3 protein is a member of the cysteine-aspartic acid protease (caspase) family. Sequential activation of caspases plays a central role in the execution-phase of cell apoptosis. Caspases exist as inactive proenzymes that undergo proteolytic processing at conserved aspartic residues to produce two subunits, large and small, that dimerize to form the active enzyme. This protein cleaves and activates caspases 6 and 7; and the protein itself is processed and activated by caspases 8, 9, and 10. It is the predominant caspase involved in the cleavage of amyloid-beta 4A precursor protein, which is associated with neuronal death in Alzheimer's disease. Alternative splicing of this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylation
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP :ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Tyrosine Phosphatase
Protein tyrosine phosphatases (EC 3.1.3.48, systematic name protein-tyrosine-phosphate phosphohydrolase) are a group of enzymes that remove phosphate groups from phosphorylated tyrosine residues on proteins: : proteintyrosine phosphate + H2O = proteintyrosine + phosphate Protein tyrosine (pTyr) phosphorylation is a common post-translational modification that can create novel recognition motifs for protein interactions and cellular localization, affect protein stability, and regulate enzyme activity. As a consequence, maintaining an appropriate level of protein tyrosine phosphorylation is essential for many cellular functions. Tyrosine-specific protein phosphatases (PTPase; ) catalyse the removal of a phosphate group attached to a tyrosine residue, using a cysteinyl-phosphate enzyme intermediate. These enzymes are key regulatory components in signal transduction pathways (such as the MAP kinase pathway) and cell cycle control, and are important in the control of cell growth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ephrin-A3
Ephrin A3 is a protein that in humans is encoded by the ''EFNA3'' gene. This gene encodes a member of the ephrin (EPH) family. The ephrins and EPH-related receptors comprise the largest subfamily of receptor protein-tyrosine kinases and have been implicated in mediating developmental events, especially in the nervous system and in erythropoiesis. Based on their structures and sequence relationships, ephrins are divided into the ephrin-A (EFNA) class, which are anchored to the membrane by a glycosylphosphatidylinositol linkage, and the ephrin-B (EFNB) class, which are transmembrane proteins. This gene encodes an EFNA class ephrin. References Further reading * * * * * * * * * * * * * {{gene-1-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leptin
Leptin (from Greek λεπτός ''leptos'', "thin" or "light" or "small") is a hormone predominantly made by adipose cells and enterocytes in the small intestine that helps to regulate energy balance by inhibiting hunger, which in turn diminishes fat storage in adipocytes. Leptin is coded for by the ''LEP'' gene. Leptin acts on cell receptors in the arcuate and ventromedial nuclei, as well as other parts of the hypothalamus and dopaminergic neurons of the ventral tegmental area, consequently mediating feeding. Although regulation of fat stores is deemed to be the primary function of leptin, it also plays a role in other physiological processes, as evidenced by its many sites of synthesis other than fat cells, and the many cell types beyond hypothalamic cells that have leptin receptors. Many of these additional functions are yet to be fully defined. In obesity, a decreased sensitivity to leptin occurs (similar to insulin resistance in type 2 diabetes), resulting in an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tumor Necrosis Factor-α
Tumor necrosis factor (TNF, cachexin, or cachectin; formerly known as tumor necrosis factor alpha or TNF-α) is an adipokine and a cytokine. TNF is a member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain. As an adipokine, TNF promotes insulin resistance, and is associated with obesity-induced type 2 diabetes. As a cytokine, TNF is used by the immune system for cell signaling. If macrophages (certain white blood cells) detect an infection, they release TNF to alert other immune system cells as part of an inflammatory response. TNF signaling occurs through two receptors: TNFR1 and TNFR2. TNFR1 is constituitively expressed on most cell types, whereas TNFR2 is restricted primarily to endothelial, epithelial, and subsets of immune cells. TNFR1 signaling tends to be pro-inflammatory and apoptotic, whereas TNFR2 signaling is anti-inflammatory and promotes cell proliferation. Suppression of TNFR1 signaling has been important for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin
Interleukins (ILs) are a group of cytokines (secreted proteins and signal molecules) that are expressed and secreted by white blood cells (leukocytes) as well as some other body cells. The human genome encodes more than 50 interleukins and related proteins. The function of the immune system primarily depends on interleukins, and rare deficiencies of a number of them have been described, all featuring autoimmune diseases or immune deficiency. The majority of interleukins are synthesized by CD4 helper T-lymphocyte, as well as through monocytes, macrophages, and endothelial cells. They promote the development and differentiation of T and B lymphocytes, and hematopoietic cells. Interleukin receptors on astrocytes in the hippocampus are also known to be involved in the development of spatial memories in mice. History and name The name "interleukin" was chosen in 1979, to replace the various different names used by different research groups to designate interleukin 1 (ly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ischemia
Ischemia or ischaemia is a restriction in blood supply to any tissue, muscle group, or organ of the body, causing a shortage of oxygen that is needed for cellular metabolism (to keep tissue alive). Ischemia is generally caused by problems with blood vessels, with resultant damage to or dysfunction of tissue i.e. hypoxia and microvascular dysfunction. It also implies local hypoxia in a part of a body resulting from constriction (such as vasoconstriction, thrombosis, or embolism). Ischemia causes not only insufficiency of oxygen, but also reduced availability of nutrients and inadequate removal of metabolic wastes. Ischemia can be partial (poor perfusion) or total blockage. The inadequate delivery of oxygenated blood to the organs must be resolved either by treating the cause of the inadequate delivery or reducing the oxygen demand of the system that needs it. For example, patients with myocardial ischemia have a decreased blood flow to the heart and are prescribed wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apoptosis
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes ( morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay. The average adult human loses between 50 and 70 billion cells each day due to apoptosis. For an average human child between eight and fourteen years old, approximately twenty to thirty billion cells die per day. In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism's life cycle. For example, the separation of fingers and toes in a developing human embryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |