|
Mineral Physics
Mineral physics is the science of materials that compose the interior of planets, particularly the Earth. It overlaps with petrophysics, which focuses on whole-rock properties. It provides information that allows interpretation of surface measurements of seismic waves, gravity anomalies, geomagnetic fields and electromagnetic fields in terms of properties in the deep interior of the Earth. This information can be used to provide insights into plate tectonics, mantle convection, the geodynamo and related phenomena. Laboratory work in mineral physics require high pressure measurements. The most common tool is a diamond anvil cell, which uses diamonds to put a small sample under pressure that can approach the conditions in the Earth's interior. Creating high pressures Shock compression Many of the pioneering studies in mineral physics involved explosions or projectiles that subject a sample to a shock. For a brief time interval, the sample is under pressure as the shock wav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage".Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica". Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral patter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plastics, and other materials. The atoms/molecules in the sintered material diffuse across the boundaries of the particles, fusing the particles together and creating a solid piece. Since the sintering temperature does not have to reach the melting point of the material, sintering is often chosen as the shaping process for materials with extremely high melting points, such as tungsten and molybdenum. The study of sintering in metallurgy, metallurgical powder-related processes is known as powder metallurgy. An example of sintering can be observed when ice cubes in a glass of water adhere to each other, which is driven by the temperature difference between the water and the ice. Examples of pressure-driven sintering are the compacting of snowfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argon
Argon is a chemical element; it has symbol Ar and atomic number 18. It is in group 18 of the periodic table and is a noble gas. Argon is the third most abundant gas in Earth's atmosphere, at 0.934% (9340 ppmv). It is more than twice as abundant as water vapor (which averages about 4000 ppmv, but varies greatly), 23 times as abundant as carbon dioxide (400 ppmv), and more than 500 times as abundant as neon (18 ppmv). Argon is the most abundant noble gas in Earth's crust, comprising 0.00015% of the crust. Nearly all argon in Earth's atmosphere is radiogenic argon-40, derived from the decay of potassium-40 in Earth's crust. In the universe, argon-36 is by far the most common argon isotope, as it is the most easily produced by stellar nucleosynthesis in supernovas. The name "argon" is derived from the Greek word , neuter singular form of meaning 'lazy' or 'inactive', as a reference to the fact that the element undergoes almost no chemical reactions. The complete oc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron transfer, Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one Substrate (chemistry), substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage
Voltage, also known as (electrical) potential difference, electric pressure, or electric tension, is the difference in electric potential between two points. In a Electrostatics, static electric field, it corresponds to the Work (electrical), work needed per unit of Electric charge, charge to move a positive Test particle#Electrostatics, test charge from the first point to the second point. In the SI unit, International System of Units (SI), the SI derived unit, derived unit for voltage is the ''volt'' (''V''). The voltage between points can be caused by the build-up of electric charge (e.g., a capacitor), and from an electromotive force (e.g., electromagnetic induction in a Electric generator, generator). On a macroscopic scale, a potential difference can be caused by electrochemical processes (e.g., cells and batteries), the pressure-induced piezoelectric effect, and the thermoelectric effect. Since it is the difference in electric potential, it is a physical Scalar (physics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule Heating
Joule heating (also known as resistive heating, resistance heating, or Ohmic heating) is the process by which the passage of an electric current through a conductor (material), conductor produces heat. Joule's first law (also just Joule's law), also known in countries of the former Soviet Union, USSR as the Joule–Lenz law, Assuming the element behaves as a perfect resistor and that the power is completely converted into heat, the formula can be re-written by substituting Ohm's law, V = I R , into the generalized power equation: P = IV = I^2R = V^2/R where ''R'' is the electrical resistance and conductance, resistance. Voltage can be increased in DC circuits by connecting batteries or solar panels in series. Alternating current When current varies, as it does in AC circuits, P(t) = U(t) I(t) where ''t'' is time and ''P'' is the instantaneous active power being converted from electrical energy to heat. Far more often, the ''average'' power is of more interest than the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity
The thermal conductivity of a material is a measure of its ability to heat conduction, conduct heat. It is commonly denoted by k, \lambda, or \kappa and is measured in W·m−1·K−1. Heat transfer occurs at a lower rate in materials of low thermal conductivity than in materials of high thermal conductivity. For instance, metals typically have high thermal conductivity and are very efficient at conducting heat, while the opposite is true for insulating materials such as mineral wool or Styrofoam. Metals have this high thermal conductivity due to free electrons facilitating heat transfer. Correspondingly, materials of high thermal conductivity are widely used in heat sink applications, and materials of low thermal conductivity are used as thermal insulation. The reciprocal of thermal conductivity is called thermal resistivity. The defining equation for thermal conductivity is \mathbf = - k \nabla T, where \mathbf is the heat flux, k is the thermal conductivity, and \nabla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transparency And Translucency
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property of allowing light to pass through the material without appreciable scattering of light. On a macroscopic scale (one in which the dimensions are much larger than the wavelengths of the photons in question), the photons can be said to follow Snell's law. Translucency (also called translucence or translucidity) is the physical property of allowing light to pass through the material (with or without scattering of light). It allows light to pass through but the light does not necessarily follow Snell's law on the macroscopic scale; the photons may be scattered at either of the two interfaces, or internally, where there is a change in the index of refraction. In other words, a translucent material is made up of components with different indices of refraction. A transparent material is made up of components with a uniform index of refraction. Transparent materials appear clear, with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion (mechanical), abrasion. In general, different materials differ in their hardness; for example hard metals such as titanium and beryllium are harder than soft metals such as sodium and metallic tin, or wood and common plastics. Macroscopic hardness is generally characterized by strong intermolecular bonds, but the behavior of solid materials under force is complex; therefore, hardness can be measured in different ways, such as scratch hardness, indentation hardness, and rebound hardness. Hardness is dependent on ductility, elasticity (physics), elastic stiffness, plasticity (physics), plasticity, deformation (mechanics), strain, strength of materials, strength, toughness, viscoelasticity, and viscosity. Common examples of hard matter are cer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
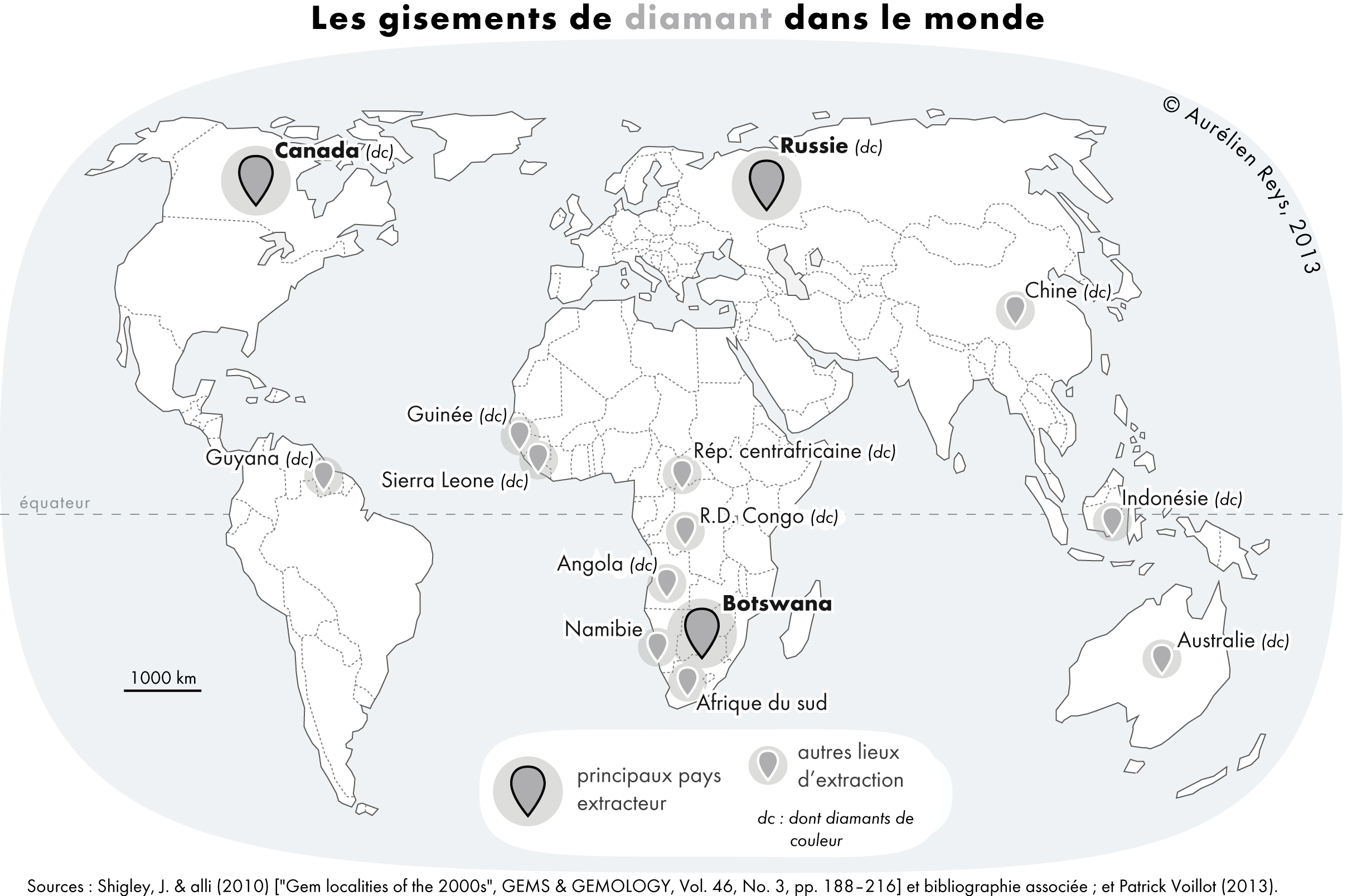
Diamonds
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of electricity, and insoluble in water. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, but diamond is metastable and converts to it at a negligible rate under those conditions. Diamond has the highest hardness and thermal conductivity of any natural material, properties that are used in major industrial applications such as cutting and polishing tools. Because the arrangement of atoms in diamond is extremely rigid, few types of impurity can contaminate it (two exceptions are boron and nitrogen). Small numbers of defects or impurities (about one per million of lattice atoms) can color a diamond blue (boron), yellow (nitrogen), brown (defects), green (radiation exposure), purple, pink, oran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inner Core
Earth's inner core is the innermost internal structure of Earth, geologic layer of the planet Earth. It is primarily a solid ball (mathematics), ball with a radius of about , which is about 20% of Earth's radius or 70% of the Moon's radius. There are no samples of the core accessible for direct measurement, as there are for Earth's mantle. The characteristics of the core have been deduced mostly from measurements of seismic waves and Earth's magnetic field. The inner core is believed to be composed of an iron–nickel alloy with some other elements. The temperature at its surface is estimated to be approximately , about the temperature at the surface of the Sun. The inner core is solid at high temperature because of its high pressure, in accordance with the Simon-Glatzel equation. Scientific history Earth was discovered to have a solid inner core distinct from its molten Earth's outer core in 1936, by the Danish seismologist Inge Lehmann's study of seismograms from earthqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Physics Today
''Physics Today'' is the membership magazine of the American Institute of Physics. First published in May 1948, it is issued on a monthly schedule, and is provided to the members of ten physics societies, including the American Physical Society. It is also available to non-members as a paid annual subscription. The magazine informs readers about important developments in overview articles written by experts, shorter review articles written internally by staff, and also discusses issues and events of importance to the science community in politics, education, and other fields. The magazine provides a historical resource of events associated with physics. For example it discussed debunking the physics of the Star Wars program of the 1980s, and the state of physics in China and the Soviet Union during the 1950s and 1970s. According to the ''Journal Citation Reports'', the journal has a 2017 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic jou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |