|
Microchannel Reactor
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm; the most typical form of such confinement are microchannels. Microreactors are studied in the field of micro process engineering, together with other devices (such as micro heat exchangers) in which physical processes occur. The microreactor is usually a continuous flow reactor (contrast with/to a batch reactor). Microreactors can offer many advantages over conventional scale reactors, including improvements in energy efficiency, reaction speed and yield, safety, reliability, scalability, on-site/on-demand production, and a much finer degree of process control. History Gas-phase microreactors have a long history but those involving liquids started to appear in the late 1990s. One of the first microreactors with embedded high performance heat exchangers were made in the early 1990s by the Central Ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrex
Pyrex (trademarked as ''PYREX'' and ''pyrex'') is a brand introduced by Corning Inc. in 1915, initially for a line of clear, low-thermal-expansion borosilicate glass used for laboratory glassware and kitchenware. It was later expanded in the 1930s to include kitchenware products made of soda–lime glass and other materials. Its name has become famous for making rectangular glass roasters. In 1998, the kitchenware division of Corning Inc. responsible for the development of Pyrex spun off from its parent company as Corning Consumer Products Company, subsequently renamed Corelle Brands. Corning Inc. no longer manufactures or markets consumer products, only industrial ones. History Borosilicate glass was first made by German chemist and glass technologist Otto Schott, founder of Schott AG in 1893, 22 years before Corning produced the Pyrex brand. Schott AG sells the product under the name "Duran". In 1908, Eugene Sullivan, director of research at Corning Inc., Corning Glass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
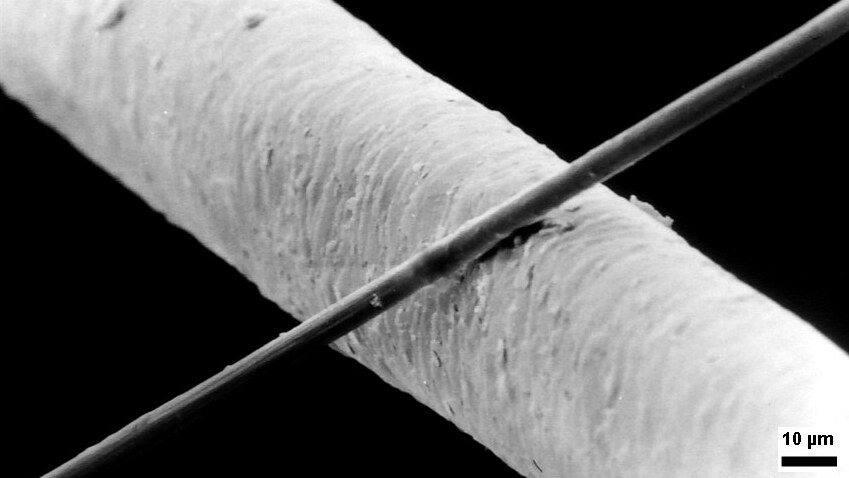
Micrometre
The micrometre (English in the Commonwealth of Nations, Commonwealth English as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer (American English), also commonly known by the non-SI term micron, is a unit of length in the International System of Units (SI) equalling (SI standard prefix "micro-" = ); that is, one millionth of a metre (or one thousandth of a millimetre, , or about ). The nearest smaller common SI Unit, SI unit is the nanometre, equivalent to one thousandth of a micrometre, one millionth of a millimetre or one billionth of a metre (). The micrometre is a common unit of measurement for wavelengths of infrared radiation as well as sizes of biological cell (biology), cells and bacteria, and for grading wool by the diameter of the fibres. The width of a single human hair ranges from approximately 20 to . Examples Between 1 μm and 10 μm: * 1–10 μm – length of a typical bacterium * 3–8 μm – width of str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are distinguished from microparticles (1-1000 μm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the Brownian motion, they usually do not sediment, like colloid, colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nanoparticles cannot be seen with ordinary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electroosmotic Flow
In chemistry, electro-osmotic flow (EOF, hyphen optional; synonymous with electro-osmosis or electro-endosmosis) is the motion of liquid induced by an applied potential across a porous material, capillary tube, membrane, microchannel, or any other fluid conduit. Because electro-osmotic velocities are independent of conduit size, as long as the electrical double layer is much smaller than the characteristic length scale of the channel, electro-osmotic flow will have little effect. Electro-osmotic flow is most significant when in small channels, and is an essential component in chemical separation techniques, notably capillary electrophoresis. Electro-osmotic flow can occur in natural unfiltered water, as well as buffered solutions. History Electro-osmotic flow was first reported in 1807 by Ferdinand Friedrich Reuss (18 February 1778 (Tübingen, Germany) – 14 April 1852 (Stuttgart, Germany)) in an unpublished lecture before the Physical-Medical Society of Moscow; Reuss firs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work-up (chemistry)
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a chemical reaction. The term is used colloquially to refer to these manipulations, which may include: * deactivating any unreacted reagents by quenching a reaction. * cooling the reaction mixture or adding an '' antisolvent'' to induce precipitation, and collecting or removing the solids by filtration, decantation, or centrifugation. * changing the protonation state of the products or impurities by adding an acid or base. * separating the reaction mixture into organic and aqueous layers by liquid-liquid extraction. * removal of solvents by evaporation. * purification by chromatography, distillation or recrystallization. The work-up steps required for a given chemical reaction may require one or more of these manipulations. Work-up steps are not always explicitly shown in reaction schemes. Written experimental procedures will describe work-up steps but will usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Operating Temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the device function and application context, and ranges from the minimum operating temperature to the maximum operating temperature (or peak operating temperature). Outside this range of safe operating temperatures the device may fail. It is one component of reliability engineering. Similarly, biological systems have a viable temperature range, which might be referred to as an "operating temperature". Ranges Most semiconductor devices are manufactured in several temperature grades. Broadly accepted grades are: *Commercial: 0 °C to 70 °C () *Industrial: −40 °C to 85 °C () *Military: −55 °C to 125 °C () Nevertheless, each manufacturer defines its own temperature grades so designers must pay attention to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a reaction occurs but in itself tells nothing about its rate. Chemical kinetics includes investigations of how experimental conditions influence the speed of a chemical reaction and yield information about the reaction's mechanism and transition states, as well as the construction of mathematical models that also can describe the characteristics of a chemical reaction. History The pioneering work of chemical kinetics was done by German chemist Ludwig Wilhelmy in 1850. He experimentally studied the rate of inversion of sucrose and he used integrated rate law for the determination of the reaction kinetics of this reaction. His work was noticed 34 years later by Wilhelm Ostwald. In 1864, Peter Waage and Cato Guldberg published the law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic
In thermodynamics, an exothermic process () is a thermodynamic process or reaction that releases energy from the system to its surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). The term ''exothermic'' was first coined by 19th-century French chemist Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy, usually in the form of heat. The concept is frequently applied in the physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Two types of chemical reactions Exothermic and endothermic describe two types of chemical reactions or systems found in nature, as follows: Exothermic An exothermic reaction occurs when heat is released to the surroundings. According to the IUPAC, an exothermic reaction is "a reaction for which the overall stand ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |