|
Methyl Cyanoacrylate
Methyl cyanoacrylate (MCA; also sometimes referred to as α-cyanoacrylate or alpha-cyanoacrylate) is an organic compound that contains several functional groups: a methyl ester, a nitrile, and an alkene. It is a colorless liquid with low viscosity. Its chief use is as the main component of cyanoacrylate glues. It can be encountered under many trade names. Methyl cyanoacrylate is less commonly encountered than ethyl cyanoacrylate. It is soluble in acetone, methyl ethyl ketone, nitromethane, and dichloromethane. MCA polymerizes rapidly in presence of moisture. Safety Heating the polymer causes depolymerization of the cured MCA, producing gaseous products which are a strong irritant to the lungs and eyes. With regard to occupational exposure to MCA, the National Institute for Occupational Safety and Health The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Cyanoacrylate
Ethyl cyanoacrylate (ECA), a cyanoacrylate ester, is an ethyl ester of 2-cyano-acrylic acid. It is a colorless liquid with low viscosity and a faint sweet smell in pure form. It is the main component of cyanoacrylate glues and can be encountered under many trade names. It is soluble in acetone, methyl ethyl ketone, nitromethane, and methylene chloride. ECA polymerizes rapidly in presence of moisture. Production Ethyl cyanoacrylate is prepared by the condensation of formaldehyde with ethyl cyanoacetate: : This exothermic reaction affords the polymer, which is subsequently sintered, thermally "cracked" to give the monomer. Alternatively, it can be prepared by the ethoxycarbonylation of cyanoacetylene. Applications Ethyl cyanoacrylate is used for gluing. In forensics, cyanoacrylate ester has excellent non-destructive impressioning abilities, which are especially important when lifting fingerprints from delicate evidence items, or when the prints could not be lifted using tradi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a characteristic pungent odor. Acetone is miscibility, miscible with properties of water, water and serves as an important organic solvent in industry, home, and laboratory. About 6.7 million tonnes were produced worldwide in 2010, mainly for use as a solvent and for production of methyl methacrylate and bisphenol A, which are precursors to widely used plastics.Acetone World Petrochemicals report, January 2010Stylianos Sifniades, Alan B. Levy, "Acetone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. It is a common building block in organic chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanoacrylate Esters
Cyanoacrylates are a family of strong fast-acting adhesives with industrial, medical, and household uses. They are derived from ethyl cyanoacrylate and related esters. The cyanoacrylate group in the monomer rapidly polymerizes in the presence of water to form long, strong chains. Specific cyanoacrylates include methyl 2-cyanoacrylate (MCA), ethyl 2-cyanoacrylate (ECA, commonly sold under trade names such as "Super Glue" and "Krazy Glue"), ''n''-butyl cyanoacrylate (n-BCA), octyl cyanoacrylate, and 2-octyl cyanoacrylate (used in medical, veterinary and first aid applications). Cyanoacrylate adhesives are sometimes known generically as instant glue, power glue, or super glue. The abbreviation "CA" is commonly used for industrial grade cyanoacrylate. Development The original patent for cyanoacrylate was filed in 1947 by the B.F. Goodrich Company as an outgrowth of a search for materials suitable for clear plastic gun sights for the war effort. In 1942, a team of scientist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Esters
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom chemical bond, bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In chemical formula, formulas, the group is often skeletal formula#Pseudoelement symbols, abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (chemistry), radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Institute For Occupational Safety And Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting research and making recommendations for the prevention of work-related occupational injury, injury, occupational disease, illness, disability, and occupational fatality, death. Its functions include gathering information, conducting scientific research both in the laboratory and in the field, and translating the knowledge gained into products and services.About NIOSH National Institute for Occupational Safety and Health. Among NIOSH's programs are determination of recommended exposure limits for toxic chemicals and other hazards, field research such as the Health Hazard Evaluation Program, epidemiology and health surveillance programs such as the National Firefighter Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Occupational Safety And Health
Occupational safety and health (OSH) or occupational health and safety (OHS) is a multidisciplinary field concerned with the safety, health, and welfare of people at work (i.e., while performing duties required by one's occupation). OSH is related to the fields of occupational medicine and occupational hygiene and aligns with workplace health promotion initiatives. OSH also protects all the general public who may be affected by the occupational environment. According to the official estimates of the United Nations, the '' WHO/ ILO'' ''Joint Estimate of the Work-related Burden of Disease and Injury'', almost 2 million people die each year due to exposure to occupational risk factors. Globally, more than 2.78 million people die annually as a result of workplace-related accidents or diseases, corresponding to one death every fifteen seconds. There are an additional 374 million non-fatal work-related injuries annually. It is estimated that the economic burden of o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
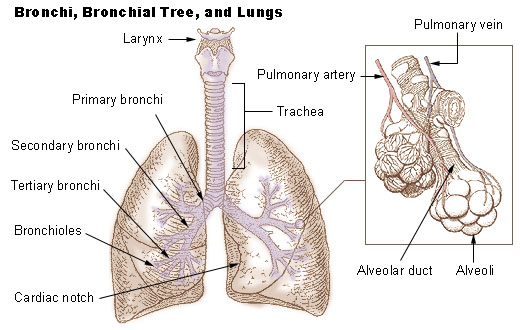
Lung
The lungs are the primary Organ (biology), organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the Vertebral column, backbone on either side of the heart. Their function in the respiratory system is to extract oxygen from the atmosphere and transfer it into the bloodstream, and to release carbon dioxide from the bloodstream into the atmosphere, in a process of gas exchange. Respiration is driven by different muscular systems in different species. Mammals, reptiles and birds use their musculoskeletal systems to support and foster breathing. In early tetrapods, air was driven into the lungs by the pharyngeal muscles via buccal pumping, a mechanism still seen in amphibians. In humans, the primary muscle that drives breathing is the Thoracic diaphragm, diaphragm. The lungs also provide airflow that makes Animal communication#Auditory, vocalisation including speech possible. Humans have two lungs, a ri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depolymerization
Depolymerization (or depolymerisation) is the process of converting a polymer into a monomer or a mixture of monomers. This process is driven by an increase in entropy. Ceiling temperature The tendency of polymers to depolymerize is indicated by their ceiling temperature. At this temperature, the enthalpy of polymerization matches the entropy gained by converting a large molecule into monomers. Above the ceiling temperature, the rate of depolymerization is greater than the rate of polymerization, which inhibits the formation of the given polymer. Applications Depolymerization is a very common process. Digestion of food involves depolymerization of macromolecules, such as proteins. It is relevant to Plastic recycling, polymer recycling. Sometimes the depolymerization is well behaved, and clean monomers can be reclaimed and reused for making new plastic. In other cases, such as polyethylene, depolymerization gives a mixture of products. These products are, for polyethylene, e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Moisture
Moisture is the presence of a liquid, especially water, often in trace amounts. Moisture is defined as water in the adsorbed or absorbed phase. Small amounts of water may be found, for example, in the air (humidity), in foods, and in some commercial products. Moisture also refers to the amount of water vapor present in the air. The soil also includes moisture. Moisture control in products Control of moisture in products can be a vital part of the process of the product. There is a substantial amount of moisture in what seems to be dry matter. Ranging in products from cornflake cereals to laundry detergent, washing powders, moisture can play an important role in the final quality of the product. There are two main aspects of concern in moisture control in products: allowing too much moisture or too little of it. For example, adding some water to cornflake cereal, which is sold by weight, reduces costs and prevents it from tasting too dry, but adding too much water can affect th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PVC), which are produced in high tonnages each year due to their usefulnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dichloromethane
Dichloromethane (DCM, methylene chloride, or methylene bichloride) is an organochlorine compound with the formula . This colorless, volatile liquid with a chloroform-like, sweet odor is widely used as a solvent. Although it is not miscible with water, it is slightly polar, and miscible with many organic solvents.Rossberg, M. ''et al.'' (2006) "Chlorinated Hydrocarbons" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. . Occurrence Natural sources of dichloromethane include oceanic sources, macroalgae, wetlands, and volcanoes. However, the majority of dichloromethane in the environment is the result of industrial emissions. Production DCM is produced by treating either chloromethane or methane with chlorine gas at 400–500 °C. At these temperatures, both methane and chloromethane undergo a series of reactions producing progressively more chlorinated products. In this way, an estimated 400,000 tons were produced in the US, Europe, and Japan in 199 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitromethane
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula . It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft. Preparation Nitromethane is produced industrially by combining propane and nitric acid in the gas phase at . This exothermic reaction produces the four industrially significant nitroalkanes: nitromethane, nitroethane, 1-nitropropane, and 2-nitropropane. The reaction involves free radicals, including the alkoxyl radicals of the type , whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |