|
Mercury Oxide
Mercury oxide can refer to: * Mercury(I) oxide (mercurous oxide), Hg2O * Mercury(II) oxide (mercuric oxide), HgO See also *Montroydite, the mineral form of mercury(II) oxide {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(I) Oxide
Mercury(I) oxide, also known as mercurous oxide, is an inorganic metal oxide with the chemical formula Hg2O. It is a brown/black powder, insoluble in water but soluble in nitric acid. With hydrochloric acid, it reacts to form calomel, Hg2Cl2. Mercury(I) oxide is toxic but without taste or smell. It is chemically unstable and converts to mercury(II) oxide Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is ... and mercury metal. References Oxides Mercury(I) compounds Inorganic compounds {{Inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
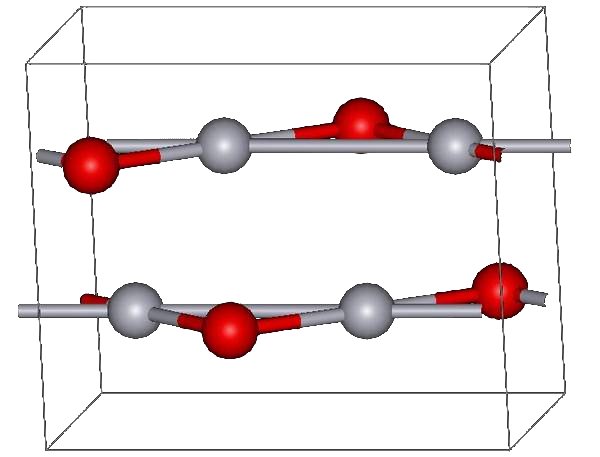
Mercury(II) Oxide
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found. History An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' It was historically called red precipitate (as opposed to white precepitate being the mercuric amidochloride). In 1774, Joseph Priestley discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen (rather, Priestley called it " dephlogisticated air," as that was the paradigm that he was working under at the time). Synthesis and reactions The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2. The yellow form can be obtained by precipitation of aque ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |