|
Manganese Carbonate
Manganese carbonate is a compound with the chemical formula Mn CO3. Manganese carbonate occurs naturally as the mineral rhodochrosite but it is typically produced industrially. It is a pale pink, water-insoluble solid. Approximately 20,000 metric tonnes were produced in 2005. Structure and production 180px, left, Manganese carbonate crystallizes in the same dense motif as calcium carbonate. Color code: red = O, green = Mn. MnCO3 adopts a structure like calcite, consisting of manganese(II) ions in an octahedral coordination geometry. Treatment of aqueous solutions of manganese(II) nitrate with ammonia and carbon dioxide leads to precipitation of this faintly pink solid. The side product, ammonium nitrate is used as fertilizer. Reactions and uses The carbonate is insoluble in water but, like most carbonates, hydrolyses upon treatment with acids to give water-soluble salts. Manganese carbonate decomposes with release of carbon dioxide, i.e. calcining, at 200 °C to gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Deficiency (medicine)
Manganese deficiency in humans results in several medical problems. Manganese is a vital element of nutrition in very small quantities (adult male daily intake 2.3 milligrams). However, poisoning may occur when greater amounts are ingested. Function Manganese is a component of some enzymes (such as arginase) and stimulates the development and activity of other enzymes. Manganese superoxide dismutase (MnSOD) is the principal antioxidant in mitochondria. Several enzymes activated by manganese contribute to the metabolism of carbohydrates, amino acids, and cholesterol. A deficiency of manganese causes skeletal deformation in animals and inhibits the production of collagen in wound healing. Food sources Manganese is found in leafy green vegetables, fruits, nuts, cinnamon, and whole grains. The nutritious kernel, called wheat germ, which contains the most minerals and vitamins of the grain, has been removed from most processed grains (such as white bread). The wheat germ is oft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganism
Manganism or manganese poisoning is a toxic condition resulting from chronic exposure to manganese. It was first identified in 1837 by James Couper. Signs and symptoms Chronic exposure to excessive manganese levels can lead to a variety of psychiatric and motor disturbances, termed manganism. Generally, exposure to ambient manganese air concentrations in excess of 5 mg/m3 can lead to manganese-induced symptoms. In initial stages of manganism, neurological symptoms consist of reduced response speed, irritability, mood changes, and compulsive behaviors. Upon protracted exposure symptoms are more prominent and resemble those of idiopathic Parkinson's disease, as which it is often misdiagnosed, although there are particular differences in both the symptoms; for example, the nature of the tremors, response to drugs such as levodopa, and affected portion of the basal ganglia. Symptoms are also similar to Lou Gehrig's disease and multiple sclerosis. Causes Welding Manganism has beco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decorative Concrete
Decorative concrete is the use of concrete as not simply a utilitarian medium for construction but as an aesthetic enhancement to a structure, while still serving its function as an integral part of the building itself such as floors, walls, driveways, and patios. The transformation of concrete into decorative concrete is achieved through the use of a variety of materials that may be applied during the pouring process or after the concrete is cured, these materials and/or systems include but are not limited to stamped concrete, acid staining, decorative overlays, polished concrete, concrete countertops, vertical overlays and more. Concrete statues Concrete statues are made by pouring concrete into a latex mold. Often painted and sold for garden ornaments. Most popular designs are bird baths and garden gnomes. Stamped concrete As a modern technique, stamped concrete is the process of adding texture and color to concrete to make it resemble stone, brick, slate, cobblestone a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic Colorants
{{nofootnotes, date=November 2010Ceramic colorants are added to a glaze or a clay to create color. Carbonates and oxides of certain metals, characterize most colorants including the commonly used cobalt carbonate, cobalt oxide, chrome oxide, red iron oxide, and copper carbonate. These colorants can create a multitude of colors depending on other materials they interact with and to which temperature and in which atmosphere they are fired. Cobalt Cobalt is commonly used in either its carbonate (CoCO3) or its oxide (Co3O4) forms. In the presence of most fluxes, it yields blue colors ranging from low saturation pastels to high saturation midnight blues in both oxidation and reduction atmospheres. However, in the presence of magnesium, cobalt can become purple or pink depending on whether it was fired in oxidation (yields purple) or reduction. Cobalt is also commonly used in black glazes and in washes as decorative medium. Common saturation percentages for low saturation range from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic Glaze
Ceramic glaze, or simply glaze, is a glassy coating on ceramics. It is used for decoration, to ensure the item is impermeable to liquids and to minimize the adherence of pollutants. Glazing renders earthenware impermeable to water, sealing the inherent porosity of earthenware. It also gives a tougher surface. Glaze is also used on stoneware and porcelain. In addition to their functionality, glazes can form a variety of surface finishes, including degrees of glossy or matte finish and color. Glazes may also enhance the underlying design or texture either unmodified or inscribed, carved or painted. Most pottery produced in recent centuries has been glazed, other than pieces in bisque porcelain, terracotta, and some other types. Tiles are often glazed on the surface face, and modern architectural terracotta is often glazed. Glazed brick is also common. Sanitaryware is invariably glazed, as are many ceramics used in industry, for example ceramic insulators for overhead power li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
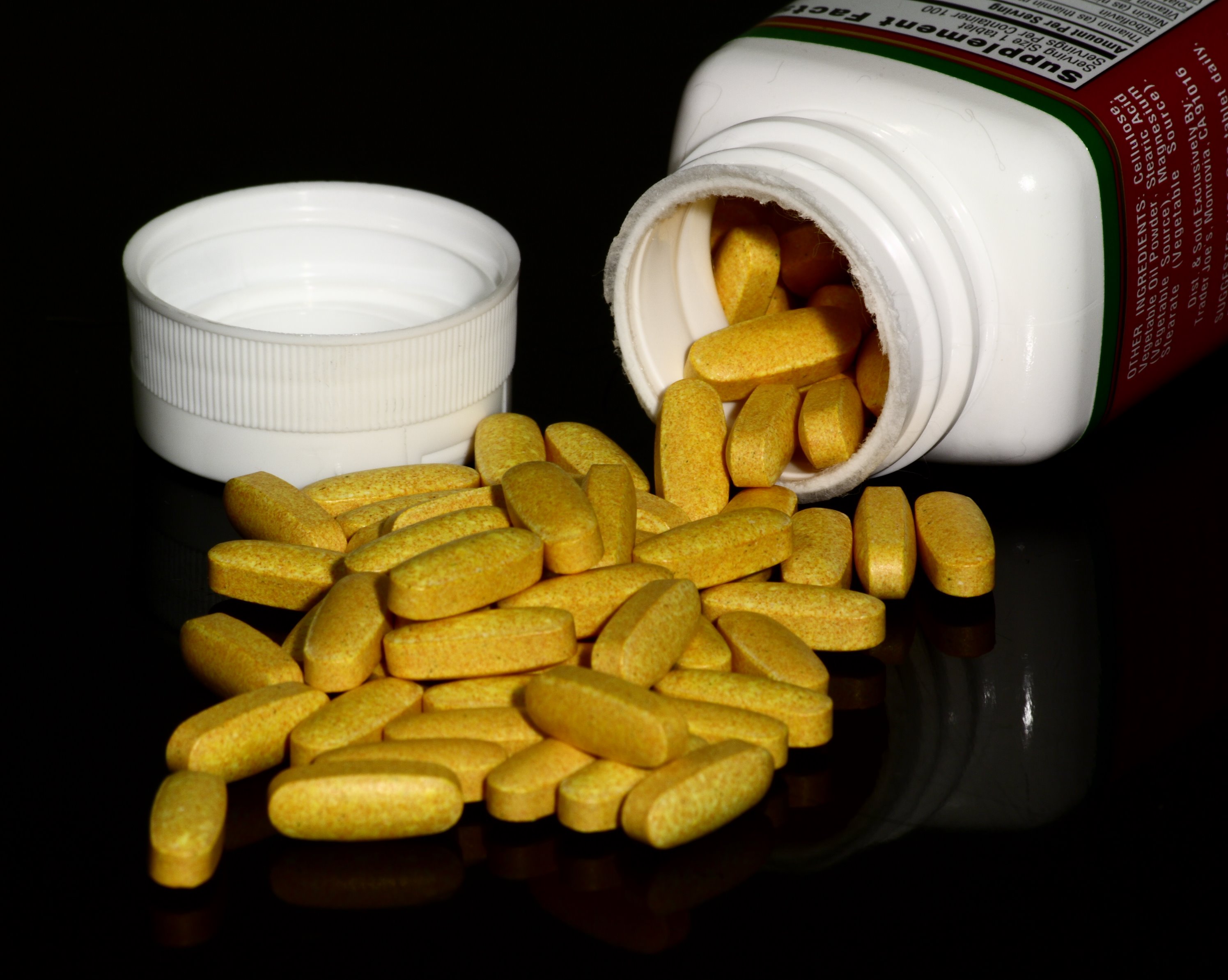
Multivitamin
A multivitamin is a preparation intended to serve as a dietary supplement with vitamins, dietary minerals, and other nutritional elements. Such preparations are available in the form of tablets, capsules, pastilles, powders, liquids, gummies, or injectable formulations. Other than injectable formulations, which are only available and administered under medical supervision, multivitamins are recognized by the Codex Alimentarius Commission (the United Nations' authority on food standards) as a category of food. In healthy people, most scientific evidence indicates that multivitamin supplements do not prevent cancer, heart disease, or other ailments, and regular supplementation is not necessary. However, specific groups of people may benefit from multivitamin supplements, for example, people with poor nutrition or those at high risk of macular degeneration, and women who are pregnant or trying to get pregnant. There is no standardized scientific definition for multivitamin. In t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrite (magnet)
A ferrite is one of a family of iron oxide-containing magnetic ceramic materials. They are ferrimagnetic, meaning they are attracted by magnetic fields and can be Magnetization, magnetized to become permanent magnets. Unlike many ferromagnetic materials, most ferrites are not electrically electrical conductor, conductive, making them useful in applications like magnetic cores for transformers to suppress eddy currents. Ferrites can be divided into two groups based on their magnetic coercivity, their resistance to being demagnetized: "Hard" ferrites have high coercivity, so are difficult to demagnetize. They are used to make permanent magnets for applications such as refrigerator magnets, loudspeakers, and small electric motors. "Soft" ferrites have low coercivity, so they easily change their magnetization and act as conductors of magnetic fields. They are used in the electronics industry to make efficient magnetic cores called ferrite cores for high-frequency inductors, transfo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Manganese Dioxide
Manganese dioxide is the inorganic compound with the formula . This blackish or brown solid occurs naturally as the mineral pyrolusite, which is the main ore of manganese and a component of manganese nodules. The principal use for is for dry-cell batteries, such as the alkaline battery and the zinc–carbon battery, although it is also used for other battery chemistries such as aqueous zinc-ion batteries.. is also used as a pigment and as a precursor to other manganese compounds, such as . It is used as a reagent in organic synthesis, for example, for the oxidation of allylic alcohols. has an α- polymorph that can incorporate a variety of atoms (as well as water molecules) in the "tunnels" or "channels" between the manganese oxide octahedra. There is considerable interest in as a possible cathode for lithium-ion batteries. Structure Several polymorphs of are claimed, as well as a hydrated form. Like many other dioxides, crystallizes in the rutile crystal structure (t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcining
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally for the purpose of removing impurities or volatile substances and/or to incur thermal decomposition. The root of the word calcination refers to its most prominent use, which is to remove carbon from limestone (calcium carbonate) through combustion to yield calcium oxide (quicklime). This calcination reaction is CaCO3(s) → CaO(s) + CO2(g). Calcium oxide is a crucial ingredient in modern cement, and is also used as a chemical flux in smelting. Industrial calcination generally emits carbon dioxide (). A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled atmosphere. Etymology The process of calcination der ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Coordination Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix '' octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |