|
Malt Sugar
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) glycosidic bond, bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germination, germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix '-ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysacc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrose
Sucrose, a disaccharide, is a sugar composed of glucose and fructose subunits. It is produced naturally in plants and is the main constituent of white sugar. It has the molecular formula . For human consumption, sucrose is extracted and refined from either sugarcane or sugar beet. Sugar mills – typically located in tropical regions near where sugarcane is grown – crush the cane and produce raw sugar which is shipped to other factories for refining into pure sucrose. Sugar beet factories are located in temperate climates where the beet is grown, and process the beets directly into refined sugar. The Sugar refinery, sugar-refining process involves washing the raw sugar crystals before dissolving them into a sugar syrup which is filtered and then passed over carbon to remove any residual colour. The sugar syrup is then concentrated by boiling under a vacuum and crystallized as the final purification process to produce crystals of pure sucrose that are clear, odorless, and sweet. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. The common dietary monosaccharides galactose, glucose and fructose are all reducing sugars. Disaccharides are formed from two monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides like sucrose and trehalose have glycosidic bonds between their anomeric carbons and thus cannot convert to an open-chain form with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
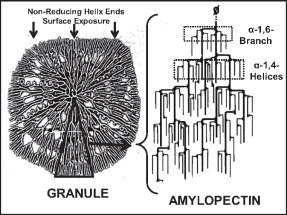
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolysis of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and Russet Burbank potato, russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Starch gelatinization, Dissolved amylopectin starch has a lower tendency of retrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycogen
Glycogen is a multibranched polysaccharide of glucose that serves as a form of energy storage in animals, fungi, and bacteria. It is the main storage form of glucose in the human body. Glycogen functions as one of three regularly used forms of energy reserves, creatine phosphate being for very short-term, glycogen being for short-term and the triglyceride stores in adipose tissue (i.e., body fat) being for long-term storage. Protein, broken down into amino acids, is seldom used as a main energy source except during starvation and glycolytic crisis ''(see bioenergetic systems)''. In humans, glycogen is made and stored primarily in the cells of the liver and skeletal muscle. In the liver, glycogen can make up 5–6% of the organ's fresh weight: the liver of an adult, weighing 1.5 kg, can store roughly 100–120 grams of glycogen. In skeletal muscle, glycogen is found in a low concentration (1–2% of the muscle mass): the skeletal muscle of an adult weighing 70 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cellobiose
Cellobiose is a disaccharide with the formula (C6H7(OH)4O)2O. It is classified as a reducing sugar - any sugar that possesses the ability or function of a reducing agent. The chemical structure of cellobiose is derived from the condensation of a pair of β-glucose molecules forming a β(1→4) bond. It can be hydrolyzed to glucose enzymatically or with acid. Cellobiose has eight free alcohol (OH) groups, one acetal linkage, and one hemiacetal linkage, which give rise to strong inter- and intramolecular hydrogen bonds. It is a white solid. It can be obtained by enzymatic or acidic hydrolysis of cellulose and cellulose-rich materials such as cotton, jute, or paper. Cellobiose can be used as an indicator carbohydrate for Crohn's disease and malabsorption syndrome. Treatment of cellulose with acetic anhydride and sulfuric acid Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), know ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule. The suffix ''-yl'' is used when naming organic compounds that contain a single bond replacing one hydrogen; ''-ylidene'' and ''-ylidyne'' are used with double bonds and triple bonds, respectively. In addition, when naming hydrocarbons that contain a substituent, positional numbers are used to indicate which carbon atom the substituent attaches to when such information is needed to distinguish between isomers. Substituents can be a combination of the inductive effect and the mesomeric effect. Such effects are also described as electron-rich and electron withdrawing. Additional steric effects result from the volume occupied by a substituent. The phrases ''most-substituted'' and ''least-substituted'' are frequently used to describe or compare molecules that are products of a chemical reaction. In this terminology, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyranose
In organic chemistry, pyranose is a collective term for saccharides that have a chemical structure that includes a six-membered ring consisting of five carbon atoms and one oxygen atom (a heterocycle). There may be other carbons external to the ring. The name derives from its similarity to the oxygen heterocycle pyran, but the pyranose ring does not have double bonds. A pyranose in which the anomeric (hydroxyl group) at C(l) has been converted into an OR group is called a pyranoside. Formation The pyranose ring is formed by the reaction of the hydroxyl group on carbon 5 (C-5) of a sugar with the aldehyde at carbon 1. This forms an intramolecular hemiacetal. If reaction is between the C-4 hydroxyl and the aldehyde, a furanose is formed instead. The pyranose form is thermodynamically more stable than the furanose form, which can be seen by the distribution of these two cyclic forms in solution. History Hermann Emil Fischer won the Nobel Prize in Chemistry (1902) fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexose
In chemistry, a hexose is a monosaccharide (simple sugar) with six carbon atoms. The chemical formula for all hexoses is , and their molecular weight is 180.156 g/mol. Hexoses exist in two forms, open-chain or cyclic, that easily convert into each other in aqueous solutions. The open-chain form of a hexose, which usually is favored in solutions, has the general structure , where ''n'' is 1, 2, 3, 4, 5. Namely, five of the carbons have one hydroxyl functional group () each, connected by a single bond, and one has an oxo group (), forming a carbonyl group (). The remaining bonds of the carbon atoms are satisfied by seven hydrogen atoms. The carbons are commonly numbered 1 to 6 starting at the end closest to the carbonyl. Hexoses are extremely important in biochemistry, both as isolated molecules (such as glucose and fructose) and as building blocks of other compounds such as starch, cellulose, and glycosides. Hexoses can form dihexose (like sucrose) by a condensation re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long-chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as hemicellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oligosaccharide
An oligosaccharide (; ) is a carbohydrate, saccharide polymer containing a small number (typically three to ten) of monosaccharides (simple sugars). Oligosaccharides can have many functions including Cell–cell recognition, cell recognition and cell adhesion. They are normally present as glycans: oligosaccharide chains are linked to lipids or to compatible amino acid side chains in proteins, by ''N''- or ''O''-glycoside, glycosidic bonds. ''N''-Linked oligosaccharides are always pentasaccharides attached to asparagine via a beta linkage to the amine nitrogen of the side chain.. Alternately, O-linked glycosylation, ''O''-linked oligosaccharides are generally attached to threonine or serine on the alcohol group of the side chain. Not all natural oligosaccharides occur as components of glycoproteins or glycolipids. Some, such as the raffinose series, occur as storage or transport carbohydrates in plants. Others, such as maltodextrins or cellodextrins, result from the microbial break ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built. Chemically, monosaccharides are polyhydroxy aldehydes with the formula or polyhydroxy ketones with the formula with three or more carbon atoms. They are usually colorless, water- soluble, and crystalline organic solids. Contrary to their name (sugars), only some monosaccharides have a sweet taste. Most monosaccharides have the formula (CH2O)''x'' (though not all molecules with this formula are monosaccharides). Examples of monosaccharides include glucose (dextrose), fructose (levulose), and galactose. Monosaccharides are the building blocks of disaccharides (such as sucrose, lactose and maltose) and polysaccharides (such as cellulose and starch). The table sugar used in everyday vernacular is itself a disaccharide sucrose comprising one molecule of each of the two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |