|
Macroscopic Limit
In statistical mechanics, the thermodynamic limit or macroscopic limit, of a system is the limit for a large number of particles (e.g., atoms or molecules) where the volume is taken to grow in proportion with the number of particles.S.J. Blundell and K.M. Blundell, "Concepts in Thermal Physics", Oxford University Press (2009) The thermodynamic limit is defined as the limit of a system with a large volume, with the particle density held fixed: : N \to \infty,\, V \to \infty,\, \frac N V =\text In this limit, macroscopic thermodynamics is valid. There, thermal fluctuations in global quantities are negligible, and all thermodynamic quantities, such as pressure and energy, are simply functions of the thermodynamic variables, such as temperature and density. For example, for a large volume of gas, the fluctuations of the total internal energy are negligible and can be ignored, and the average internal energy can be predicted from knowledge of the pressure and temperature of the g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Statistical Mechanics
In physics, statistical mechanics is a mathematical framework that applies statistical methods and probability theory to large assemblies of microscopic entities. Sometimes called statistical physics or statistical thermodynamics, its applications include many problems in a wide variety of fields such as biology, neuroscience, computer science Computer science is the study of computation, information, and automation. Computer science spans Theoretical computer science, theoretical disciplines (such as algorithms, theory of computation, and information theory) to Applied science, ..., information theory and sociology. Its main purpose is to clarify the properties of matter in aggregate, in terms of physical laws governing atomic motion. Statistical mechanics arose out of the development of classical thermodynamics, a field for which it was successful in explaining macroscopic physical properties—such as temperature, pressure, and heat capacity—in terms of microscop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Asymptotic Analysis
In mathematical analysis, asymptotic analysis, also known as asymptotics, is a method of describing Limit (mathematics), limiting behavior. As an illustration, suppose that we are interested in the properties of a function as becomes very large. If , then as becomes very large, the term becomes insignificant compared to . The function is said to be "''asymptotically equivalent'' to , as ". This is often written symbolically as , which is read as " is asymptotic to ". An example of an important asymptotic result is the prime number theorem. Let denote the prime-counting function (which is not directly related to the constant pi), i.e. is the number of prime numbers that are less than or equal to . Then the theorem states that \pi(x)\sim\frac. Asymptotic analysis is commonly used in computer science as part of the analysis of algorithms and is often expressed there in terms of big O notation. Definition Formally, given functions and , we define a binary relation f( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Flux
In electromagnetism, electric flux is the total electric field that crosses a given surface. The electric flux through a closed surface is directly proportional to the total charge contained within that surface. The electric field E can exert a force on an electric charge at any point in space. The electric field is the gradient of the electric potential. Overview An electric charge, such as a single electron in space, has an electric field surrounding it. In pictorial form, this electric field is shown as "lines of flux" being radiated from a dot (the charge). These are called Gauss lines. Note that field lines are a graphic illustration of field strength and direction and have no physical meaning as isolated lines. The density of these lines corresponds to the electric field strength, which could also be called the electric flux density: the number of "lines" per unit area. Electric flux is directly proportional to the total number of electric field lines going through a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge Density
In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density (symbolized by the Greek letter ρ) is the quantity of charge per unit volume, measured in the SI system in coulombs per cubic meter (C⋅m−3), at any point in a volume. Surface charge density (σ) is the quantity of charge per unit area, measured in coulombs per square meter (C⋅m−2), at any point on a surface charge distribution on a two dimensional surface. Linear charge density (λ) is the quantity of charge per unit length, measured in coulombs per meter (C⋅m−1), at any point on a line charge distribution. Charge density can be either positive or negative, since electric charge can be either positive or negative. Like mass density, charge density can vary with position. In classical electromagnetic theory charge density is idealized as a '' continuous'' scalar function of position \boldsymbol, like a fluid, and \rho(\boldsymbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gravitation
In physics, gravity (), also known as gravitation or a gravitational interaction, is a fundamental interaction, a mutual attraction between all massive particles. On Earth, gravity takes a slightly different meaning: the observed force between objects and the Earth. This force is dominated by the combined gravitational interactions of particles but also includes effect of the Earth's rotation. Gravity gives weight to physical objects and is essential to understanding the mechanisms responsible for surface water waves and lunar tides. Gravity also has many important biological functions, helping to guide the growth of plants through the process of gravitropism and influencing the circulation of fluids in multicellular organisms. The gravitational attraction between primordial hydrogen and clumps of dark matter in the early universe caused the hydrogen gas to coalesce, eventually condensing and fusing to form stars. At larger scales this results in galaxies and clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Force
In molecular physics and chemistry, the van der Waals force (sometimes van der Waals' force) is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules. Named after Dutch physicist Johannes Diderik van der Waals, the van der Waals force plays a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. It also underlies many properties of organic compounds and molecular solids, including their solubility in polar and non-polar media. If no other force is present, the distance between atoms at which the force becomes repulsive rather than attractive as the atoms approach one another is called the van der ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Particle Number Density
In thermodynamics, the particle number (symbol ) of a thermodynamic system is the number of constituent particles in that system. The particle number is a fundamental thermodynamic property which is conjugate to the chemical potential. Unlike most physical quantities, the particle number is a dimensionless quantity, specifically a countable quantity. It is an extensive property, as it is directly proportional to the size of the system under consideration and thus meaningful only for closed systems. A constituent particle is one that cannot be broken into smaller pieces at the scale of energy involved in the process (where is the Boltzmann constant and is the temperature). For example, in a thermodynamic system consisting of a piston containing water vapour, the particle number is the number of water molecules in the system. The meaning of constituent particles, and thereby of particle numbers, is thus temperature-dependent. Determining the particle number The concept of par ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
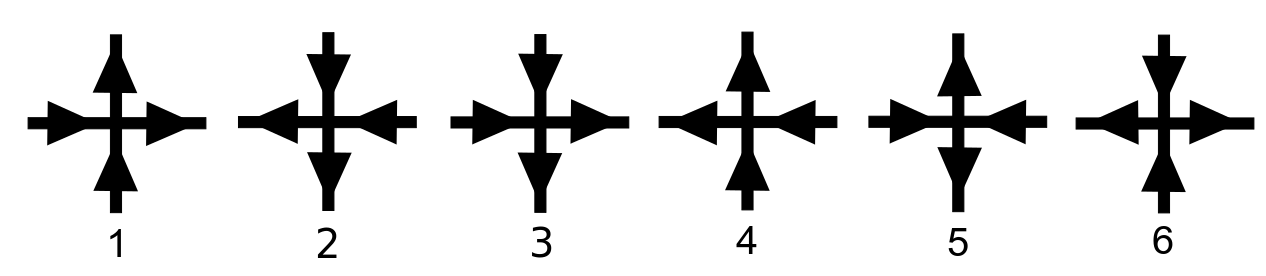
Six Vertex Model
In statistical mechanics, the ice-type models or six-vertex models are a family of vertex models for crystal lattices with hydrogen bonds. The first such model was introduced by Linus Pauling in 1935 to account for the residual entropy of water ice. Variants have been proposed as models of certain ferroelectric and antiferroelectric crystals. In 1967, Elliott H. Lieb found the exact solution to a two-dimensional ice model known as "square ice". The exact solution in three dimensions is only known for a special "frozen" state. Description An ice-type model is a lattice model defined on a lattice of coordination number 4. That is, each vertex of the lattice is connected by an edge to four "nearest neighbours". A state of the model consists of an arrow on each edge of the lattice, such that the number of arrows pointing inwards at each vertex is 2. This restriction on the arrow configurations is known as the ice rule. In graph theoretic terms, the states are Eulerian orien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extensive Quantity
Physical or chemical properties of materials and systems can often be categorized as being either intensive or extensive, according to how the property changes when the size (or extent) of the system changes. The terms "intensive and extensive quantities" were introduced into physics by German mathematician Georg Helm in 1898, and by American physicist and chemist Richard C. Tolman in 1917. According to International Union of Pure and Applied Chemistry (IUPAC), an intensive property or intensive quantity is one whose magnitude is independent of the size of the system. An intensive property is not necessarily homogeneously distributed in space; it can vary from place to place in a body of matter and radiation. Examples of intensive properties include temperature, ''T''; refractive index, ''n''; density, ''ρ''; and hardness, ''η''. By contrast, an extensive property or extensive quantity is one whose magnitude is additive for subsystems. Examples include mass, volume and Gi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grand Canonical Ensemble
In statistical mechanics, the grand canonical ensemble (also known as the macrocanonical ensemble) is the statistical ensemble that is used to represent the possible states of a mechanical system of particles that are in thermodynamic equilibrium (thermal and chemical) with a reservoir. The system is said to be open in the sense that the system can exchange energy and particles with a reservoir, so that various possible states of the system can differ in both their total energy and total number of particles. The system's volume, shape, and other external coordinates are kept the same in all possible states of the system. The thermodynamic variables of the grand canonical ensemble are chemical potential (symbol: ) and absolute temperature (symbol: . The ensemble is also dependent on mechanical variables such as volume (symbol: , which influence the nature of the system's internal states. This ensemble is therefore sometimes called the ensemble, as each of these three quantitie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canonical Ensemble
In statistical mechanics, a canonical ensemble is the statistical ensemble that represents the possible states of a mechanical system in thermal equilibrium with a heat bath at a fixed temperature. The system can exchange energy with the heat bath, so that the states of the system will differ in total energy. The principal thermodynamic variable of the canonical ensemble, determining the probability distribution of states, is the absolute temperature (symbol: ). The ensemble typically also depends on mechanical variables such as the number of particles in the system (symbol: ) and the system's volume (symbol: ), each of which influence the nature of the system's internal states. An ensemble with these three parameters, which are assumed constant for the ensemble to be considered canonical, is sometimes called the ensemble. The canonical ensemble assigns a probability to each distinct microstate given by the following exponential: :P = e^, where is the total energy of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |