|
Isoxazolidine
Isoxazolidine is the organic compound with the formula (CH2)3(NH)O. It is the parent of a family of compounds called Isoxazolidines, which are saturated C3NO heterocyclic rings where the nitrogen and oxygen occupy adjacent positions (1 and 2). They are the saturated analogues of Isoxazoles, and they are isomeric with oxazolidines, where the N and O are separated by one carbon. Isoxazolidines can be produced by the nitrone-olefin (3+2) cycloaddition reaction. : They represent precursors to 1,3-aminoalcohols. The series Organic Syntheses provides detailed procedures that yield isoxazolidines, e.g., from styrene and N-phenylmaleimide Maleimide is a chemical compound with the formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functional group. Malei .... Some isoxazolidines are of medicinal interest.{{Cite journal , doi = 10.1021/acs.chemrev.6b0054 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxazolidine
Oxazolidine is a five-membered heterocycle ringwith the formula .The O atom and NH groups are not mutually bonded, in contrast to isoxazolidine. Oxazolidines (emphasis on plural) are derivatives of the parent oxazolidine owing to the presence of substituents on carbon and/or nitrogen. Oxazolines are unsaturated analogues of oxazolidines. Synthesis and reactions First synthesized in the 1800s, oxazolidines are traditionally prepared by condensation of 2- aminoalcohols with aldehydes and ketones. The ready availability of chiral amino alcohols by reduction of amino acids enables the synthesis of chiral oxazolidines. Oxazolidines are prone to hydrolysis, the reverse of their syntheses. Perhaps for this reason, their basicity is rarely discussed. Uses and occurrence Several oxazolidine derivatives occur naturally, Some occur as post translational modifications of proteins. Others are components of alkaloids, a few of which are highly active against some tumors. Examples include ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
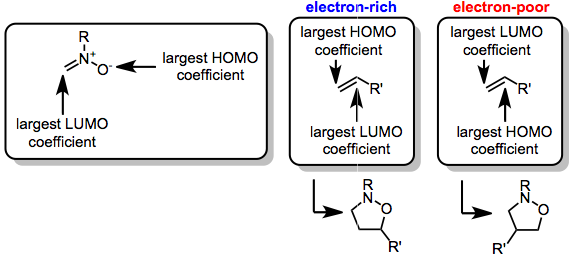
Nitrone-olefin (3+2) Cycloaddition
The nitrone-olefin (3+2) cycloaddition reaction is the combination of a nitrone with an alkene or alkyne to generate an isoxazoline or isoxazolidine via a (3+2) cycloaddition process. This reaction is a 1,3-dipolar cycloaddition, in which the nitrone acts as the 1,3-dipole, and the alkene or alkyne as the dipolarophile. Mechanism and stereochemistry When nitrones are combined with either alkenes or alkynes, (3+2) cycloaddition leads to the formation of a new C–C bond and a new C–O bond. The cycloadditions is stereospecific with respect to the configuration of the alkene; however, diastereoselectivity in reactions of C-substituted nitrones is often low. Regioselectivity is controlled by the dominant frontier orbitals interacting during the reaction, and substrates with electronically distinct substituents tend to react with high regioselectivity. Intramolecular versions of the reaction have been used to synthesize complex polyclic carbon frameworks. Reduction of the N–O lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Ring
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoxazole
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the Valence (chemistry), univalent functional group derived from isoxazole. Occurrence Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol. Synthesis Isoxazole can be synthesised via a variety of methods. Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid. Photochemistry The photolysis of isoxazole was first reported in 1966. Due to the weak N-O bond, the isoxazole ring tends to collapse under UV irradiation, rearranging to oxazole through azirine intermediate. Meanwhile, the azirine intermediate can react with nucleophiles, especially carboxylic acids. Given the photoreactions, isoxazole group is developed as a native photo-cross-linker for photoaffinity labeling and chemoproteomic studi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Syntheses
''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and experiments reported in an article must be successfully repeated in the laboratory of a member of the editorial board as a check for reproducibility prior to publication. The journal is published by Organic Syntheses, Inc., a non-profit corporation. An annual print version is published by John Wiley & Sons on behalf of Organic Syntheses, Inc. History Prior to World War I, work on synthetic organic chemistry in the United States had been quite limited, and most of the reagents used in laboratories had to be imported from Europe. When export stoppages and trade embargoes cut off this source, Clarence Derick, a professor of chemistry at University of Illinois at Urbana-Champaign, began an effort to synthesize these needed chemicals in industri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene is an organic compound with the chemical formula C6H5CH=CH2. Its structure consists of a vinyl group as substituent on benzene. Styrene is a colorless, oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers, and is typically made from benzene for this purpose. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maleimide
Maleimide is a chemical compound with the formula H2C2(CO)2NH (see diagram). This unsaturated imide is an important building block in organic synthesis. The name is a contraction of maleic acid and imide, the -C(O)NHC(O)- functional group. Maleimides are also a ''class'' of derivatives of the parent maleimide where the N''H'' group is replaced with alkyl or aryl groups such as a methyl or phenyl, respectively. The substituent can also be a small molecule (such as biotin, a fluorescent dye, an oligosaccharide, or a nucleic acid), a reactive group, or a synthetic polymer such as polyethylene glycol. Human hemoglobin chemically modified with maleimide-polyethylene glycol is a blood substitute called MP4. Reactions Many analogues of maleimide are prepared by treating maleic anhydride with amines followed by dehydration. A defining feature of the reactivity of maleimides is their susceptibility to additions across the double bond either by Michael additions or via Diels-Alder react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicinal Chemistry
Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with drug design, designing and developing pharmaceutical medication, drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entity, new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR). Medicinal chemistry is a highly interdisciplinary science combining organic chemistry with biochemistry, computational chemistry, pharmacology, molecular biology, statistics, and physical chemistry. Compounds used as medicines are most often organic compounds, which are often divided into the broad classes of Small molecule, small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and "biologic medical product, biologics" (infliximab, erythropoietin, insulin glargine), the latter of whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |