|
Isotopes Of Bohrium
Bohrium (107Bh) is an artificial element. Like all artificial elements, it has no stable isotopes, and a standard atomic weight cannot be given. The first isotope to be synthesized was 262Bh in 1981. There are 11 known isotopes ranging from 260Bh to 274Bh, and 1 isomer, 262mBh. The longest-lived isotope is 270Bh with a half-life of 2.4 minutes, although the unconfirmed 278Bh may have an even longer half-life of about 11.5 minutes. List of isotopes , -id=Bohrium-260 , 260Bh , style="text-align:right" , 107 , style="text-align:right" , 153 , 260.12144(21)# , [] , alpha decay, α , 256Db , , -id=Bohrium-261 , rowspan=2, 261Bh , rowspan=2 style="text-align:right" , 107 , rowspan=2 style="text-align:right" , 154 , rowspan=2, 261.12140(19) , rowspan=2, [] , α , 257Db , rowspan=2, (5/2−) , - , Spontaneous fission, SF (rare) , (various) , -id=Bohrium-262 , rowspan=2, 262Bh , rowspan=2 style="text-align:right" , 107 , rowspan=2 style="text-align:righ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohrium
Bohrium is a synthetic chemical element; it has symbol Bh and atomic number 107. It is named after Danish physicist Niels Bohr. As a synthetic element, it can be created in particle accelerators but is not found in nature. All known isotopes of bohrium are highly radioactive; the most stable known isotope is 270Bh with a half-life of approximately 2.4 minutes, though the unconfirmed 278Bh may have a longer half-life of about 11.5 minutes. In the periodic table, it is a d-block transactinide element. It is a member of the 7th period and belongs to the group 7 elements as the fifth member of the 6d series of transition metals. Chemistry experiments have confirmed that bohrium behaves as the heavier homologue to rhenium in group 7. The chemical properties of bohrium are characterized only partly, but they compare well with the chemistry of the other group 7 elements. Introduction History Discovery Two groups claimed discovery of the element. Evidence of bohrium was first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joint Institute For Nuclear Research
The Joint Institute for Nuclear Research (JINR, ), in Dubna, Moscow Oblast (110 km north of Moscow), Russia, is an international research center for nuclear sciences, with 5,500 staff members including 1,200 researchers holding over 1,000 Ph.D.s from eighteen countries. Most scientists are scientists of the Russian Federation. The institute has seven laboratories, each with its own specialisation: theoretical physics, high energy physics (particle physics), heavy ion physics, condensed matter physics, nuclear reactions, neutron physics, and information technology. The institute has a division to study radiation and radiobiological research and other ad hoc experimental physics experiments. Principal research instruments include a nuclotron superconductive particle accelerator (particle energy: 7 GeV), three isochronous cyclotrons (120, 145, 650 MeV), a phasitron (680 MeV) and a synchrophasotron (4 GeV). The site has a neutron fast-pulse reactor (1,500MW pulse) with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Institute Of Modern Physics
Zhejiang Institute of Modern Physics (Traditional Chinese: 浙江大學近代物理中心, Simplified Chinese: 浙江近代物理中心) is a research center for theoretical physics. It is part of the Zhejiang University, People's Republic of China. Introduction The institute was formally founded in 1991, due to the encouragement of American Nobel Prize laureate Tsung-Dao Lee and current President of Chinese Academy of Sciences Lu Yongxiang, both of whom are Zhejiang University alumni. Tsung-Dao Lee was named the first Director of the institute. The prominent leader Chen Yun also wrote (in calligraphy) the name for the institute. The facility is located on the hill of the Lingfeng (灵峰), and in the Yuquan Campus. It has a construction area of 1,000 m2. The Institute focuses on the study of quantum field theory, string theory, and high-energy physics. Current researchers including Mr. Xiaowei TANG (唐孝威, academician of CAS) also perform interdisciplinary studies of biop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
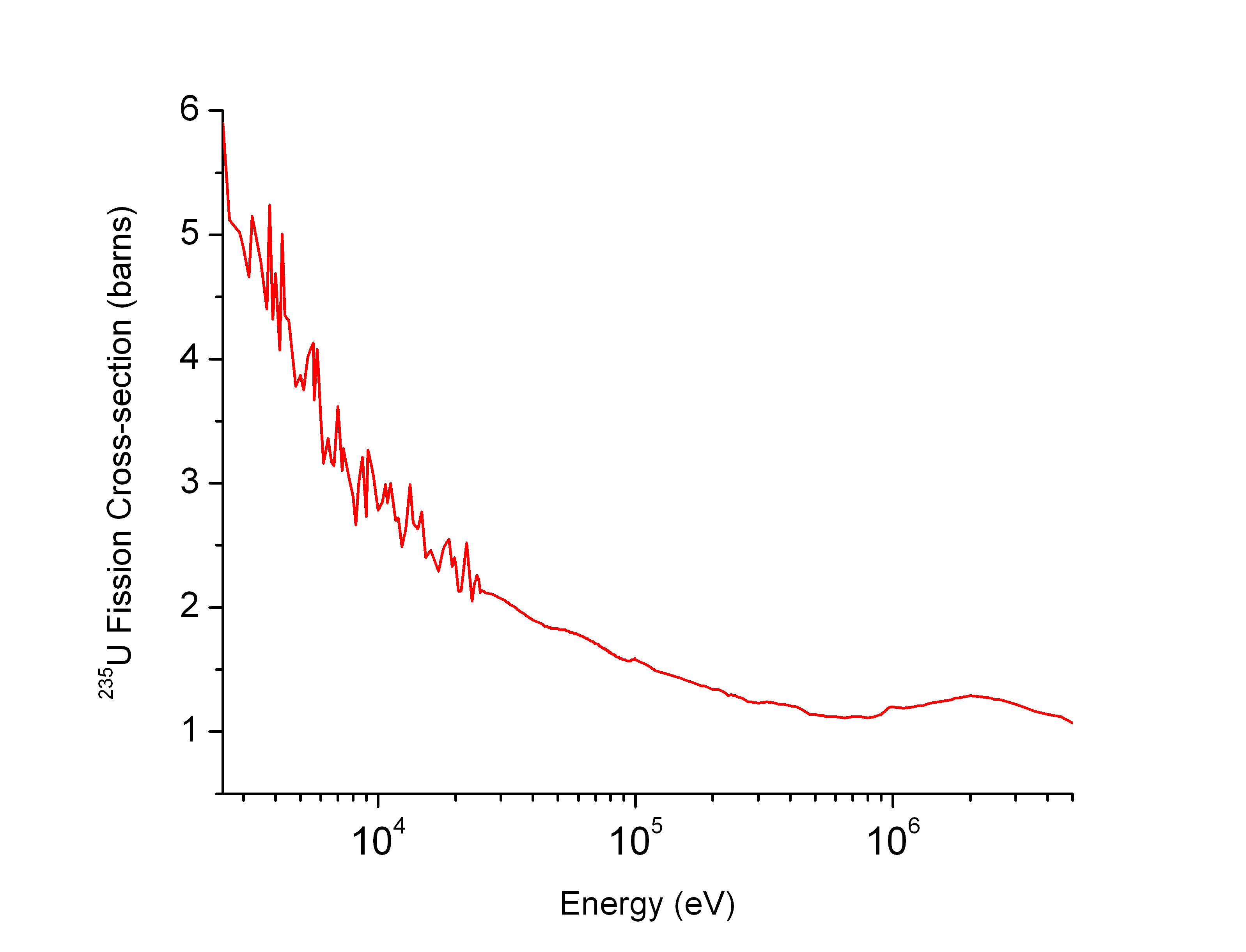
Uranium-238
Uranium-238 ( or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However, it is fissionable by fast neutrons, and is ''fertile'', meaning it can be transmuted to fissile plutonium-239. 238U cannot support a chain reaction because inelastic scattering reduces neutron energy below the range where fast fission of one or more next-generation nuclei is probable. Doppler broadening of 238U's neutron absorption resonances, increasing absorption as fuel temperature increases, is also an essential negative feedback mechanism for reactor control. Around 99.284% of natural uranium's mass is uranium-238, which has a half-life of 1.41 seconds (4.468 years, or 4.468 billion years). Due to its natural abundance and half-life relative to other radioactive elements, 238U produces ~40% of the radioactive heat produced wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cross Section (physics)
In physics, the cross section is a measure of the probability that a specific process will take place in a collision of two particles. For example, the Rutherford cross-section is a measure of probability that an alpha particle will be deflected by a given angle during an interaction with an atomic nucleus. Cross section is typically denoted (sigma) and is expressed in units of area, more specifically in barns. In a way, it can be thought of as the size of the object that the excitation must hit in order for the process to occur, but more exactly, it is a parameter of a stochastic process. When two discrete particles interact in classical physics, their mutual cross section is the area transverse to their relative motion within which they must meet in order to scatter from each other. If the particles are hard inelastic sphere A sphere (from Ancient Greek, Greek , ) is a surface (mathematics), surface analogous to the circle, a curve. In solid geometry, a sphere is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nuclear fission in 1938, the first self-sustaining nuclear reactor (Chicago Pile-1, 1942) and the first nuclear weapon (Trinity (nuclear test), Trinity, 1945). Neutrons are found, together with a similar number of protons in the atomic nucleus, nuclei of atoms. Atoms of a chemical element that differ only in neutron number are called isotopes. Free neutrons are produced copiously in nuclear fission and nuclear fusion, fusion. They are a primary contributor to the nucleosynthesis of chemical elements within stars through fission, fusion, and neutron capture processes. Neutron stars, formed from massive collapsing stars, consist of neutrons at the density of atomic nuclei but a total mass more than the Sun. Neutron properties and interactions ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Excitation Function
Excitation function ( yield curve) is a term used in nuclear physics to describe a graphical plot of the yield of a radionuclide or reaction channel as a function of the bombarding projectile energy or the calculated excitation energy of the compound nucleus. The yield is the measured intensity of a particular transition. The excitation function typically resembles a Gaussian bell curve and is mathematically described by a Breit–Wigner function, owing to the resonant nature of the production of the compound nucleus. The energy value at the maximum yield on the excitation curve corresponds to the energy of the resonance. The energy interval between 25% and 75% of the maximum yield on the excitation curve are equivalent to the resonance width. A nuclear reaction In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two atomic nucleus, nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decay Product
In nuclear physics, a decay product (also known as a daughter product, daughter isotope, radio-daughter, or daughter nuclide) is the remaining nuclide left over from radioactive decay. Radioactive decay often proceeds via a sequence of steps ( decay chain). For example, 238U decays to 234Th which decays to 234mPa which decays, and so on, to 206Pb (which is stable): : \ce \overbrace^\ce left, upThe decay chain from lead-212 down to lead-208, showing the intermediate decay products In this example: * 234Th, 234mPa,...,206Pb are the decay products of 238U. * 234Th is the daughter of the parent 238U. * 234mPa (234 metastable) is the granddaughter of 238U. These might also be referred to as the daughter products of 238U. (''Depleted Uranium'' — authors: Naomi H. Harley, Ernest C. Foulkes, Lee H. Hilb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Number
The neutron number (symbol ''N'') is the number of neutrons in a nuclide. Atomic number (proton number) plus neutron number equals mass number: . The difference between the neutron number and the atomic number is known as the neutron excess: . Neutron number is not written explicitly in nuclide symbol notation, but can be inferred as it is the difference between the two left-hand numbers (atomic number and mass). Nuclides that have the same neutron number but different proton numbers are called isotones. This word was formed by replacing the p in isotope with n for neutron. Nuclides that have the same mass number are called isobars. Nuclides that have the same neutron excess are called isodiaphers. Chemical properties are primarily determined by proton number, which determines which chemical element the nuclide is a member of; neutron number has only a slight influence. Neutron number is primarily of interest for nuclear properties. For example, actinides with odd neutron ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL, Berkeley Lab) is a Federally funded research and development centers, federally funded research and development center in the Berkeley Hills, hills of Berkeley, California, United States. Established in 1931 by the University of California (UC), the laboratory is sponsored by the United States Department of Energy and administered by the UC system. Ernest Lawrence, who won the Nobel prize for inventing the cyclotron, founded the lab and served as its director until his death in 1958. Located in the Berkeley Hills, the lab overlooks the campus of the University of California, Berkeley. Scientific research The mission of Berkeley Lab is to bring science solutions to the world. The research at Berkeley Lab has four main themes: discovery science, energy, earth systems, and the future of science. The Laboratory's 22 scientific divisions are organized within six areas of research: Computing Sciences, Physical Sciences, Earth and Environmenta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth(III) Fluoride
Bismuth(III) fluoride or bismuth trifluoride is a chemical compound of bismuth and fluorine. The chemical formula is BiF3. It is a grey-white powder melting at 649 °C. It occurs in nature as the rare mineral gananite. Synthesis Bismuth fluoride can be prepared by reacting bismuth(III) oxide with hydrofluoric acid: :Bi2O3 + 6 HF → 2 BiF3 + 3 H2O Structure α-BiF3 has a cubic crystalline structure (Pearson symbol cF16, space group Fm-3m, No. 225). BiF3 is the prototype for the D03 structure, which is adopted by several intermetallics, including Mg3Pr, Cu3Sb, Fe3Si, and AlFe3, as well as by the hydride LaH3.0. The unit cell is face-centered cubic with Bi at the face centers and vertices, and F at the octahedral site (mid-edges, center), and tetrahedral sites (centers of the 8 sub cubes) - thus the primitive cell contains 4 Bi and 12 F. Alternatively, with the unit cell shifted (1/4,1/4,1/4) the description can be of a fcc cell with face, edge, corner, and centers filled w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |