|
Isostere
Classical Isosteres are molecules or ions with similar shape and often electronic properties. Many definitions are available. but the term is usually employed in the context of bioactivity and drug development. Such biologically-active compounds containing an isostere is called a bioisostere. This is frequently used in drug design: the bioisostere will still be recognized and accepted by the body, but its functions there will be altered as compared to the parent molecule. History and additional definitions Non-classical isosteres do not obey the above classifications, but they still produce similar biological effects in vivo. Non-classical isosteres may be made up of similar atoms, but their structures do not follow an easily definable set of rules. The isostere concept was formulated by Irving Langmuir in 1919, and later modified by Grimm. Hans Erlenmeyer extended the concept to biological systems in 1932.Mukesh Doble, Anil Kumar Kruthiventi, Vilas Gajanan. ''Biotransformations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioisostere
In medicinal chemistry, bioisosteres are chemical substituents or groups with similar physical or chemical properties which produce broadly similar biological properties in the same chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or physical properties of a compound without making significant changes in chemical structure. The main use of this term and its techniques are related to pharmaceutical sciences. Bioisosterism is used to reduce toxicity, change bioavailability, or modify the activity of the lead compound, and may alter the metabolism of the lead. Examples Classical bioisosteres Classical bioisosterism was originally formulated by James Moir and refined by Irving Langmuir as a response to the observation that different atoms with the same valence electron structure had similar biological properties. For example, the replacement of a hydrogen atom with a fluorine atom at a site of metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans Erlenmeyer
Hans Friedrich Albrecht Erlenmeyer (March 20, 1900, in Strasbourg – May 29, 1967, in Basel) was a German-Swiss chemist and collector of antiquities. He was a professor of inorganic chemistry at the University of Basel. Life and career Hans Erlenmeyer came from a family of chemists; his grandfather Emil Erlenmeyer and his father Emil Erlenmeyer Jr. were both chemistry professors. Erlenmeyer studied chemistry from 1918 to 1922 at the Friedrich Schiller University of Jena and the Humboldt University of Berlin, where he received his doctorate in 1922 with a thesis entitled "On Asymmetric Synthesis". He worked as an assistant to Bernhard Lepsius. In 1925, he became an assistant to Friedrich Fichter in Basel, and in 1927 he habilitated in inorganic chemistry. He became an associate professor in 1933, and in 1941 he succeeded Fichter as full professor and head of the Institute of Inorganic Chemistry. Erlenmeyer was involved in approximately 500 publications on structural chemistry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
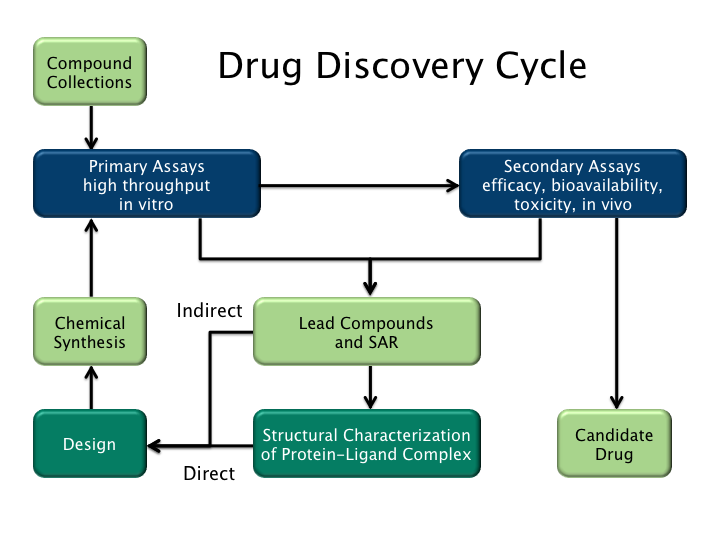
Drug Design
Drug design, often referred to as rational drug design or simply rational design, is the invention, inventive process of finding new medications based on the knowledge of a biological target. The drug is most commonly an organic compound, organic small molecule that activates or inhibits the function of a biomolecule such as a protein, which in turn results in a therapeutic effect, therapeutic benefit to the patient. In the most basic sense, drug design involves the design of molecules that are complementary in shape and electric charge, charge to the biomolecular target with which they interact and therefore will bind to it. Drug design frequently but not necessarily relies on molecular modelling, computer modeling techniques. This type of modeling is sometimes referred to as computer-aided drug design. Finally, drug design that relies on the knowledge of the three-dimensional structure of the biomolecular target is known as structure-based drug design. In addition to small molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ions
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− (chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Vivo
Studies that are ''in vivo'' (Latin for "within the living"; often not italicized in English) are those in which the effects of various biological entities are tested on whole, living organisms or cells, usually animals, including humans, and plants, as opposed to a tissue extract or dead organism. Examples of investigations ''in vivo'' include: the pathogenesis of disease by comparing the effects of bacterial infection with the effects of purified bacterial toxins; the development of non-antibiotics, antiviral drugs, and new drugs generally; and new surgical procedures. Consequently, animal testing and clinical trials are major elements of ''in vivo'' research. ''In vivo'' testing is often employed over ''in vitro'' because it is better suited for observing the overall effects of an experiment on a living subject. In drug discovery, for example, verification of efficacy ''in vivo'' is crucial, because ''in vitro'' assays can sometimes yield misleading results with drug c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Irving Langmuir
Irving Langmuir (; January 31, 1881 – August 16, 1957) was an American chemist, physicist, and metallurgical engineer. He was awarded the Nobel Prize in Chemistry in 1932 for his work in surface chemistry. Langmuir's most famous publication is the 1919 article "The Arrangement of Electrons in Atoms and Molecules" in which, building on Gilbert N. Lewis's cubical atom theory and Walther Kossel's chemical bonding theory, he outlined his "concentric theory of atomic structure". Langmuir became embroiled in a priority dispute with Lewis over this work; Langmuir's presentation skills were largely responsible for the popularization of the theory, although the credit for the theory itself belongs mostly to Lewis. While at General Electric from 1909 to 1950, Langmuir advanced several fields of physics and chemistry, inventing the gas-filled incandescent lamp and the hydrogen welding technique. The Langmuir Laboratory for Atmospheric Research near Socorro, New Mexico, was named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
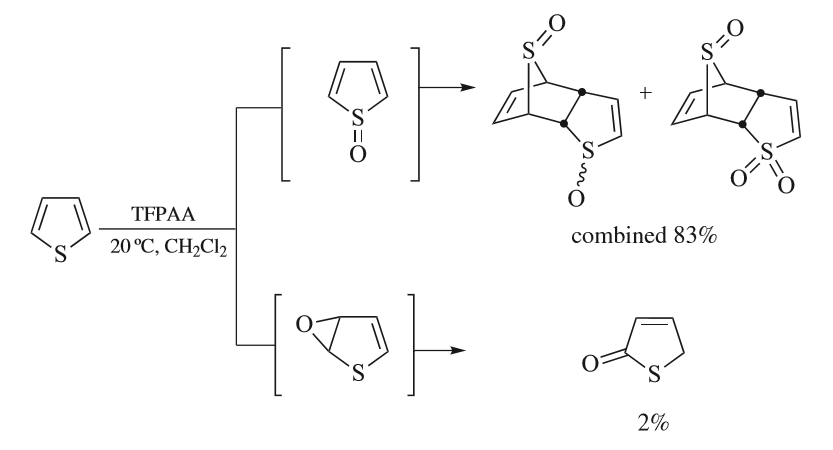
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered by Viktor Meyer in 1882 as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly Volatility (chemistry), volatile liquid with a boiling point close to room temperature. It is soluble in common organic Solvent, solvents, including ethanol, alcohol, diethyl ether, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom . It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated Polymer, polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties Pyridine is diamagnetism, diamagnetic. Its critical point (thermodynamics), critical parameters are: pressure 5.63 MPa, temperature 619 K and volume 248 cm3/mol. In the temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Theoretical Chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface of potential energy, molecular orbitals, orbital interactions, and molecule activation. Overview Theoretical chemistry unites principles and concepts common to all branches of chemistry. Within the framework of theoretical chemistry, there is a systematization of chemical laws, principles and rules, their refinement and detailing, the construction of a hierarchy. The central place in theoretical chemistry is occupied by the doctrine of the interconnection of the structure and properties of molecular systems. It uses mathematical and physical methods to explain the structures and dynamics of chemical systems and to correlate, understand, and predict their thermodynamic and kinetic properties. In the most general sense, it is explanation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |