|
Ion Exchange Resin
An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange, that is also known as an ionex. It is an solubility, insoluble matrix (or support structure) normally in the form of small (0.25–1.43 mm radius) microbeads, usually white or yellowish, fabricated from an organic chemistry, organic polymer substrate. The beads are typically porosity, porous (with a specific size distribution that will affect its properties), providing a large surface area on and inside them where the trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin, that differ in composition if the target is an anion or a cation and are created based on the task they are required for. Most commercial resins are made of polystyrene sulfonateFrançois Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion Exchange Resin Beads
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ (potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− (chloride ion) and OH− (hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indicates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polystyrene
Polystyrene (PS) is a synthetic polymer made from monomers of the aromatic hydrocarbon styrene. Polystyrene can be solid or foamed. General-purpose polystyrene is clear, hard, and brittle. It is an inexpensive resin per unit weight. It is a poor barrier to air and water vapor and has a relatively low melting point. Polystyrene is one of the most widely used plastics, with the scale of its production being several million tonnes per year. Polystyrene is naturally transparent to visible light, but can be colored with colorants. Uses include protective packaging (such as packing peanuts and optical disc jewel cases), containers, lids, bottles, trays, tumblers, disposable cutlery, in the making of models, and as an alternative material for phonograph records. As a thermoplastic polymer, polystyrene is in a solid (glassy) state at room temperature but flows if heated above about 100 °C, its glass transition temperature. It becomes rigid again when cooled. This te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PolyAPTAC
PolyAPTAC, or poly (acrylamido-N-propyltrimethylammonium chloride), is an organic polymer. It is water-soluble, forms gels when cross linked, and acts as a cationic polyelectrolyte. It can be used for ion exchange resins. It can form hydrogel A hydrogel is a Phase (matter), biphasic material, a mixture of Porosity, porous and Permeation, permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water Solubility, insoluble three dimensional network ...s. PolyMAPTAC, or poly 3-(methacryloylamino)-propyltrimethylammonium chloride), is similar. References Acrylate polymers Polyelectrolytes {{polymer-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion () and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces than bleach-based disinfectants, and are generally fabric-safe. Synthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of electrons. Amines can also exist as hetero cyclic compounds. Aniline is the simplest aromatic amine, consisting of a benzene ring bonded to an amino group. Amines are classified into three types: primary (1°), secondary (2°), and tertiary (3°) amines. Primary amines (1°) contain one alkyl or aryl substituent and have the general formula RNH2. Secondary amines (2°) have two alkyl or aryl groups attached to the nitrogen atom, with the general formula R2NH. Tertiary amines (3°) contain three substituent groups bonded to the nitrogen atom, and are represented by the formula R3N. The functional group present in primary amines is called the amino group. Classification of amines Amines can be classified according to the nature and number o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PolyAMPS
PolyAMPS, or poly(2-acrylamido-2-methyl-1-propanesulfonic acid) (trademark of the Lubrizol Corporation), is an organic polymer. It is water-soluble, forms gels when cross linked, and acts as a strong anionic polyelectrolyte. It can be used for ion exchange resins. It can form hydrogel A hydrogel is a Phase (matter), biphasic material, a mixture of Porosity, porous and Permeation, permeable solids and at least 10% of water or other interstitial fluid. The solid phase is a water Solubility, insoluble three dimensional network ...s. See also * 2-Acrylamido-2-methylpropane sulfonic acid (AMPS) References Acrylate polymers Polyelectrolytes {{polymer-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
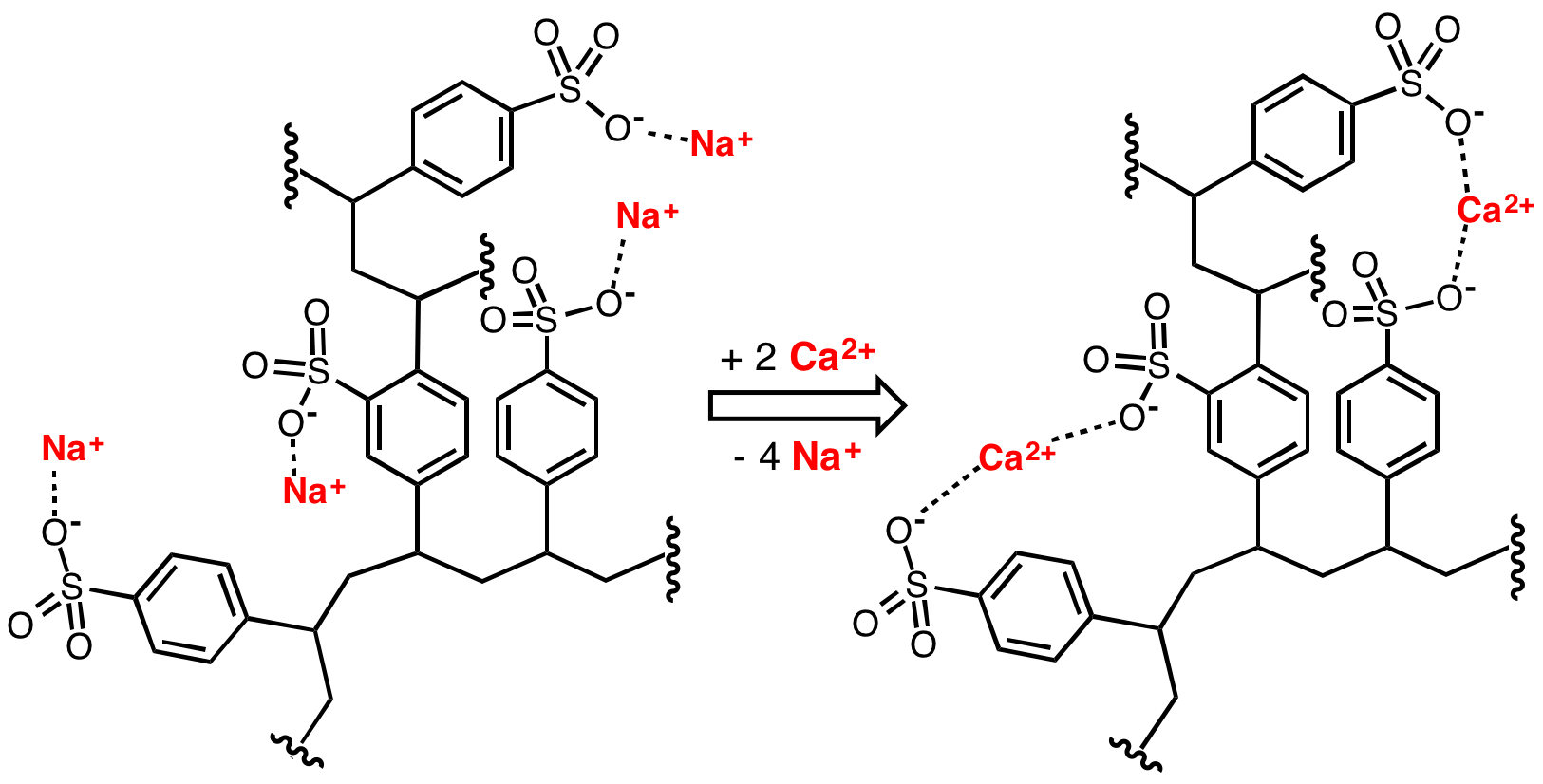
Sodium Polystyrene Sulfonate
Polystyrene sulfonates are a group of medications used to treat high blood potassium. Effects generally take hours to days. They are also used to remove potassium, calcium, and sodium from solutions in technical applications. Common side effects include loss of appetite, gastrointestinal upset, constipation, and low blood calcium. These polymers are derived from polystyrene by the addition of sulfonate functional groups. Sodium polystyrene sulfonate was approved for medical use in the United States in 1958. A polystyrene sulfonate was developed in the 2000s to treat '' Clostridioides difficile'' associated diarrhea under the name Tolevamer, but it was never marketed. Medical uses Polystyrene sulfonate is usually supplied in either the sodium or calcium form. It is used as a potassium binder in acute and chronic kidney disease for people with hyperkalemia (an abnormally high blood serum potassium level). However, it is unclear if it is beneficial and there is concern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: : In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. In a related process, carboxyli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrodialysis
Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate ( brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation-exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes (such as reverse osmosis (RO)) in that dissolved species are moved away from the feed stream, whereas other processes move away the water from the remaining substances. Because the quantity of dissolve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ion-exchange Membrane
An ion-exchange membrane is a semi-permeable membrane that transports certain dissolved ions, while blocking other ions or neutral molecules. Ion-exchange membranes are therefore electrically conductive. They are often used in desalination and chemical recovery applications, moving ions from one solution to another with little passage of water. Important examples of ion-exchange membranes include the proton-exchange membranes, that transport cations, and the anion exchange membranes used in certain alkaline fuel cells to transport anions. Structure and composition An ion-exchange membrane is generally made of organic or inorganic polymer with charged (ionic) side groups, such as ion-exchange resins. Anion-exchange membranes contain fixed cationic groups with predominantly mobile anions; because anions are the majority species, most of the conductivity is due to anion transport. The reverse holds for cation-exchange membranes. The so-called heterogeneous ion-excha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |