|
Iodotyrosine Deiodinase
Iodotyrosine deiodinase, also known as iodotyrosine dehalogenase 1, is a type of deiodinase enzyme that scavenges iodide by removing it from iodinated tyrosine residues in the thyroid gland. These iodinated tyrosines are produced during thyroid hormone biosynthesis. The iodide that is scavenged by iodotyrosine deiodinase is necessary to again synthesize the thyroid hormones. After synthesis, the thyroid hormones circulate through the body to regulate metabolic rate, protein expression, and body temperature. Iodotyrosine deiodinase is thus necessary to keep levels of both iodide and thyroid hormones in balance. Dehalogenation in aerobic organisms is usually done through Redox, oxidation and hydrolysis; however, iodotyrosine deiodinase uses reductive dehalogenation. Iodotyrosine deiodinase and iodothyronine deiodinase have been determined as the only two known enzymes to catalyze reductive dehalogenation in mammals. Although these two enzymes perform similar functions, they are st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Deiodinase
Deiodinase (monodeiodinase) is a peroxidase enzyme that is involved in the activation or deactivation of thyroid hormones. Types Types of deiodinases include: Iodothyronine deiodinases catalyze release of iodine directly from the thyronine hormones. They are selenocysteine-dependent membrane proteins with a catalytic domain resembling peroxiredoxins (Prx). Three related isoforms, deiodinase type I, II, and III, contribute to activation and inactivation of the initially released hormone precursor T4 (thyroxine) into T3 (triiodothyronine) or rT3 ( reverse triiodothyronine) in target cells. The enzymes catalyze a reductive elimination of iodine (the different isoforms attack different thyronine positions), thereby oxidizing themselves similar to Prx, followed by a reductive recycling of the enzyme. Iodotyrosine deiodinase contributes to breakdown of thyroid hormones. It releases iodine, for renewed use, from iodinated tyrosines resulting from catabolism of iodothyronines. Io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
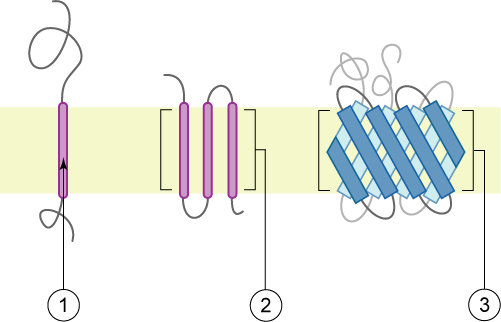
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (Transmembrane protein, transmembrane) or associate with one or the other side of a membrane (Integral monotopic protein, integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct (Native state, native) Protein structure, conformation of the protein in isolation from its native ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Goitre
A goitre (British English), or goiter (American English), is a swelling in the neck resulting from an enlarged thyroid gland. A goitre can be associated with a thyroid that is not functioning properly. Worldwide, over 90% of goitre cases are caused by iodine deficiency. The term is from the Latin ''gutturia'', meaning throat. Most goitres are not cancerous ( benign), though they may be potentially harmful. Signs and symptoms A goitre can present as a palpable or visible enlargement of the thyroid gland at the base of the neck. A goitre, if associated with hypothyroidism or hyperthyroidism, may be present with symptoms of the underlying disorder. For hyperthyroidism, the most common symptoms are associated with adrenergic stimulation: tachycardia (increased heart rate), palpitations, nervousness, tremor, increased blood pressure and heat intolerance. Clinical manifestations are often related to hypermetabolism (increased metabolism), excessive thyroid hormone, an in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
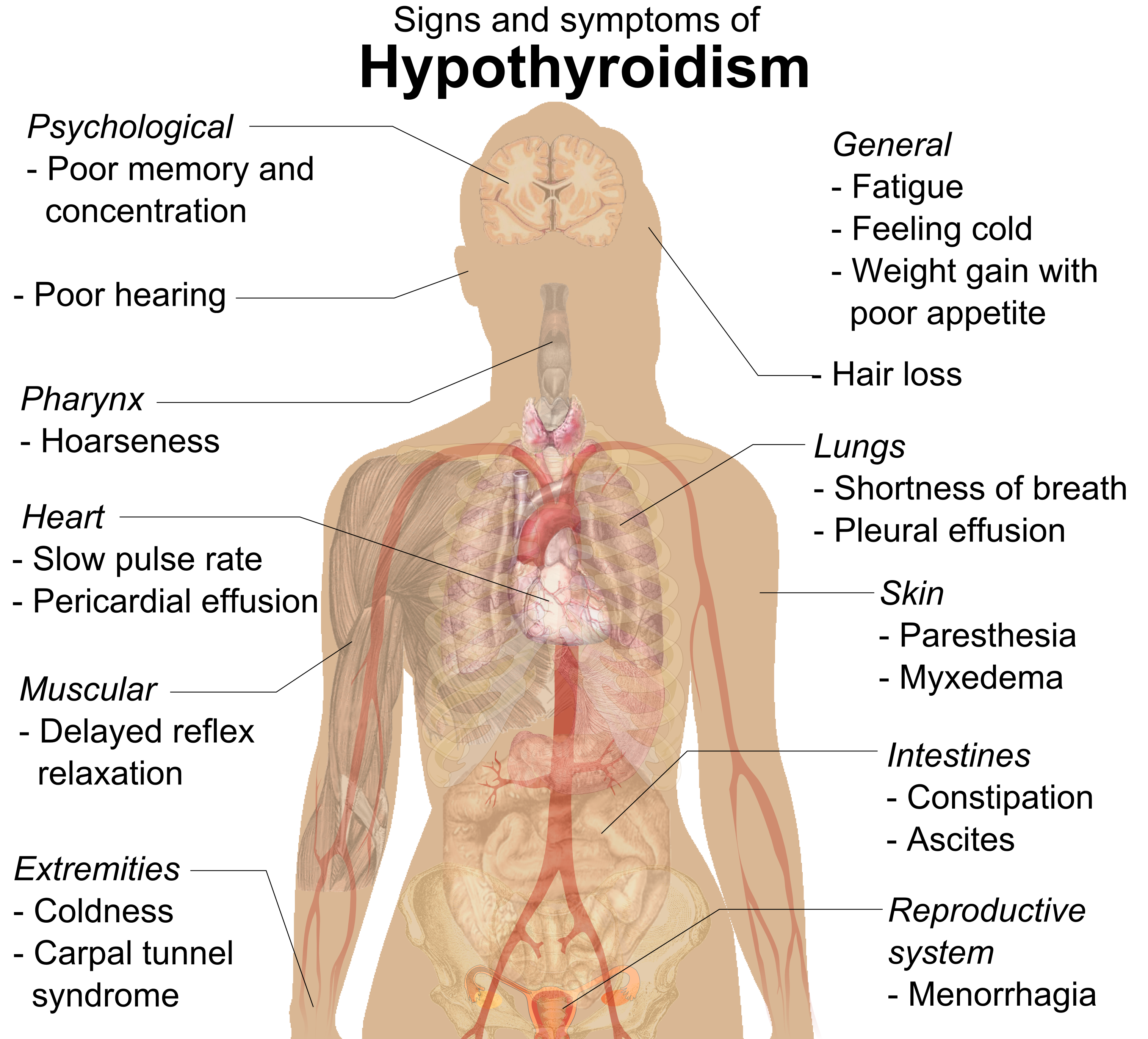
Hypothyroidism
Hypothyroidism is an endocrine disease in which the thyroid gland does not produce enough thyroid hormones. It can cause a number of symptoms, such as cold intolerance, poor ability to tolerate cold, fatigue, extreme fatigue, muscle aches, constipation, slow heart rate, Depression (mood), depression, and weight gain. Occasionally there may be swelling of the front part of the neck due to goiter. Untreated cases of hypothyroidism during pregnancy can lead to delays in child development, growth and intellectual development in the baby or congenital iodine deficiency syndrome. Worldwide, iodine deficiency, too little iodine in the diet is the most common cause of hypothyroidism. Hashimoto's thyroiditis, an autoimmune disease where the body's immune system reacts to the thyroid gland, is the most common cause of hypothyroidism in countries with sufficient dietary iodine. Less common causes include previous treatment with iodine-131, radioactive iodine, injury to the hypothalamus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Iodotyrosine Deiodinase Reaction 2
3-Iodotyrosine is an intermediate in the synthesis of thyroid hormones which is derived from iodination of tyrosine at the meta-position of the benzene ring. One unit can combine with diiodotyrosine to form triiodothyronine, as occurs in the colloid of the thyroid follicle. Two units can combine to form 3,3'-diiodothyronine. 3-Iodotyrosine is a reversible inhibitor of the enzyme tyrosine hydroxylase. Relevance in dopamine studies 3-Iodotyrosine, a pathway inhibitor in the synthesis of the neurotransmitter dopamine, was used to determine the effects of decreased dopamine levels in social spacing of ''Drosophila melanogaster ''Drosophila melanogaster'' is a species of fly (an insect of the Order (biology), order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly" ...''. 3-4 day old flies that were fed 3-iodotyrosine for 24 hours were shown to have altered dopamine l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Flavin Mononucleotide
Flavin mononucleotide (FMN), or riboflavin-5′-phosphate, is a biomolecule produced from riboflavin (vitamin B2) by the enzyme riboflavin kinase and functions as the prosthetic group of various oxidoreductases, including NADH dehydrogenase, as well as a cofactor in biological blue-light photo receptors. During the catalytic cycle, various oxidoreductases induce reversible interconversions between the oxidized (FMN), semiquinone (FMNH•), and reduced (FMNH2) forms of the isoalloxazine core. FMN is a stronger oxidizing agent than NAD and is particularly useful because it can take part in both one- and two-electron transfers. In its role as blue-light photo receptor, (oxidized) FMN stands out from the 'conventional' photo receptors as the signaling state and not an E/Z isomerization. It is the principal form in which riboflavin is found in cells and tissues. It requires more energy to produce, but is more soluble than riboflavin. In cells, FMN occurs freely circulating but ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nicotinamide Adenine Dinucleotide Phosphate
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a Cofactor (biochemistry), cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). NADPH is the redox, reduced form, whereas NADP is the redox, oxidized form. NADP is used by all forms of cellular life. NADP is essential for life because it is needed for cellular respiration. NADP differs from NAD+, NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine Moiety (chemistry), moiety. This extra phosphate is added by NAD+ kinase, NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+, NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase, NAD+ kinase adding the extra phosphate g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
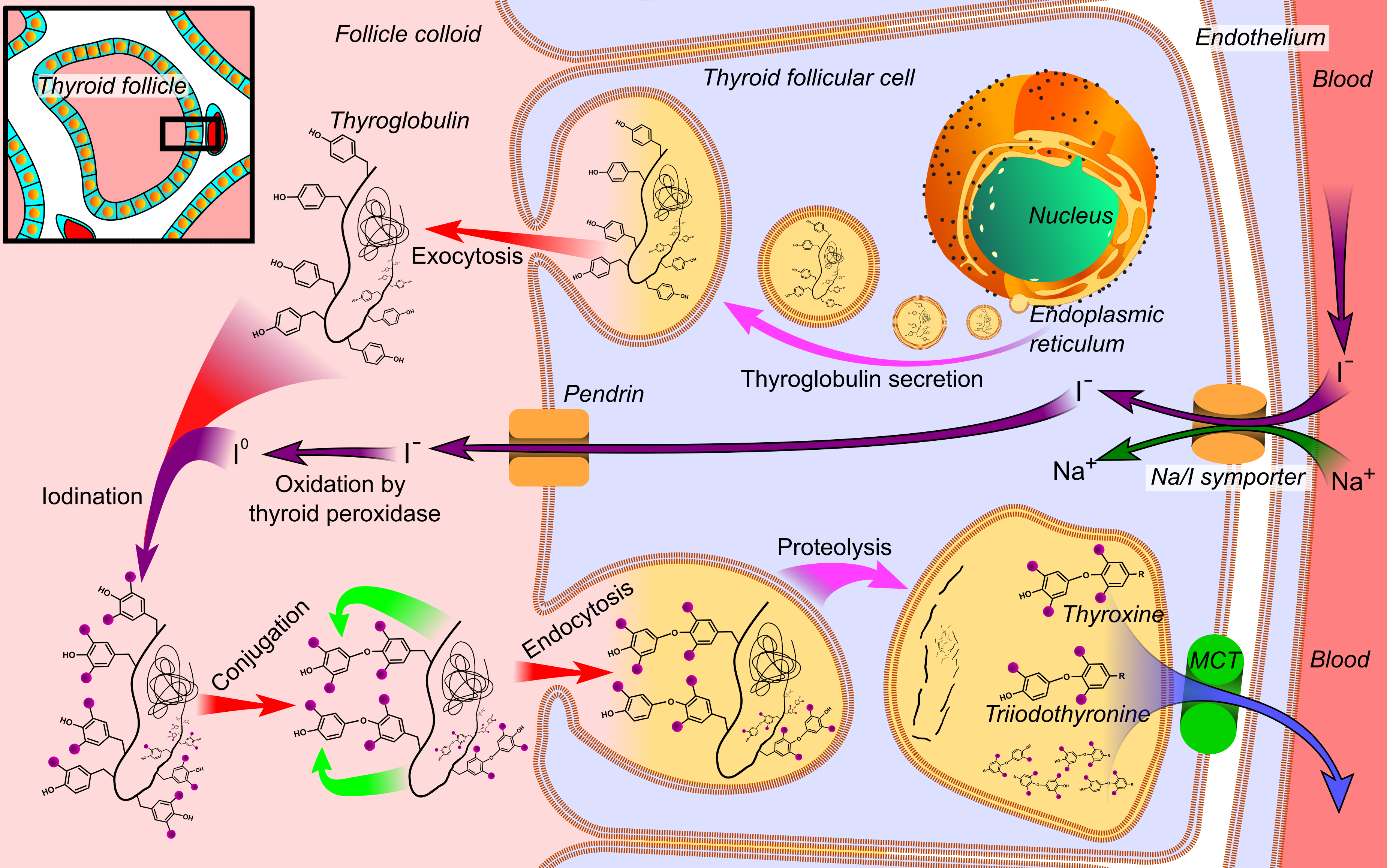
Thyroglobulin
Thyroglobulin (Tg) is a 660 kDa, dimeric glycoprotein produced by the follicular cells of the thyroid and used entirely within the thyroid gland. Tg is secreted and accumulated at hundreds of grams per litre in the extracellular compartment of the thyroid follicles, accounting for approximately half of the protein content of the thyroid gland. Human TG (hTG) is a homodimer of subunits each containing 2768 amino acids as synthesized (a short signal peptide of 19 amino acids may be removed from the N-terminus in the mature protein). Thyroglobulin is in all vertebrates the main precursor to thyroid hormones, which are produced when thyroglobulin's tyrosine residues are combined with iodine and the protein is subsequently cleaved. Each thyroglobulin molecule contains approximately 16 tyrosine residues, but only around 10 of these are subject to iodination by thyroperoxidase in the follicular colloid. It takes two iodinated tyrosines to make a thyroid hormone molecule; therefor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Homeostasis
In biology, homeostasis (British English, British also homoeostasis; ) is the state of steady internal physics, physical and chemistry, chemical conditions maintained by organism, living systems. This is the condition of optimal functioning for the organism and includes many variables, such as body temperature and fluid balance, being kept within certain pre-set limits (homeostatic range). Other variables include the pH of extracellular fluid, the concentrations of sodium, potassium, and calcium ions, as well as the blood sugar level, and these need to be regulated despite changes in the environment, diet, or level of activity. Each of these variables is controlled by one or more regulators or homeostatic mechanisms, which together maintain life. Homeostasis is brought about by a natural resistance to change when already in optimal conditions, and equilibrium is maintained by many regulatory mechanisms; it is thought to be the central motivation for all organic action. All home ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diiodotyrosine
Diiodotyrosine (DIT) is a precursor in the production of thyroid hormone, and results from iodization of monoiodotyrosine at the other meta- position on the phenol ring. Function DIT is a modulator of the enzyme thyroid peroxidase (which is involved in the production of thyroid hormones). Triiodothyronine is formed, when diiodotyrosine is combined with monoiodotyrosine (in the colloid of the thyroid follicle). Two molecules of DIT combine to make the thyroid hormone thyroxine Thyroxine, also known as T4, is a hormone produced by the thyroid gland. It is the primary form of thyroid hormone found in the blood and acts as a prohormone of the more active thyroid hormone, triiodothyronine (T3). Thyroxine and its acti ... ('T4' and 'T3'). See also * Diiodotyrosine transaminase References External links * Iodinated tyrosine derivatives {{organohalide-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Monoiodotyrosine
3-Iodotyrosine is an intermediate in the synthesis of thyroid hormones which is derived from iodination of tyrosine at the meta-position of the benzene ring. One unit can combine with diiodotyrosine to form triiodothyronine, as occurs in the colloid of the thyroid follicle. Two units can combine to form 3,3'-diiodothyronine. 3-Iodotyrosine is a reversible inhibitor of the enzyme tyrosine hydroxylase. Relevance in dopamine studies 3-Iodotyrosine, a pathway inhibitor in the synthesis of the neurotransmitter dopamine, was used to determine the effects of decreased dopamine levels in social spacing of ''Drosophila melanogaster ''Drosophila melanogaster'' is a species of fly (an insect of the Order (biology), order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly", "pomace fly" ...''. 3-4 day old flies that were fed 3-iodotyrosine for 24 hours were shown to have altered dopamine le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Active Site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding site'', and residues that catalyse a reaction of that substrate, the ''catalytic site''. Although the active site occupies only ~10–20% of the volume of an enzyme, it is the most important part as it directly catalyzes the chemical reaction. It usually consists of three to four amino acids, while other amino acids within the protein are required to maintain the tertiary structure of the enzymes. Each active site is evolved to be optimised to bind a particular substrate and catalyse a particular reaction, resulting in high specificity. This specificity is determined by the arrangement of amino acids within the active site and the structure of the substrates. Sometimes enzymes also need to bind with some cofactors to fulfil their functio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |