|
Iodoethane
Ethyl iodide (also iodoethane) is a colorless flammable chemical compound. It has the chemical formula C2H5I and is prepared by heating ethanol with iodine and phosphorus.''Merck Index of Chemicals and Drugs'', 9th ed., monograph 3753 On contact with air, especially on the effect of light, it decomposes and turns yellow or reddish from dissolved iodine. It may also be prepared by the reaction between hydroiodic acid and ethanol, typically by generating the hydroiodic acid ''in situ'' via an iodide salt (such as sodium iodide) and an acid (such as sulfuric acid), after which the ethyl iodide is distilled off. Ethyl iodide should be stored in the presence of copper powder to avoid rapid decomposition, though even with this method samples do not last more than 1 year. Because iodide is a good leaving group, ethyl iodide is an excellent ethylating agent. It is also used as the hydrogen radical promoter. Production Ethyl iodide is prepared by using red phosphorus, absolute ethanol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoform
Iodoform (also known as triiodomethane) is the organoiodine compound with the chemical formula . It is a pale yellow, crystalline, volatile substance, with a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant. Naming The name iodoform originates with the "formyle radical," an archaic term for the HC moiety, and is retained for historical consistency. A full, modern name is triiodomethane. Another possible name is "carbon hydride triiodide". The "hydride" in the latter is sometimes omitted, though the IUPAC recommends against doing so, as "carbon triiodide" could also mean (hexaiodoethane, a highly unstable compound). Structure The molecule adopts a tetrahedral molecular geometry, tetrahedral geometry with C3v symmetry group, symmetry. Synthesis and reactions The synthesis of iodofo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted in small amounts by rice plantations. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups. Preparation and handling Iodomethane is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus. The iodinating reagent is phosphorus triiodide that is formed ''in situ:'' :3 CH3OH + PI3 → 3 CH3I + H2PO3H Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodoalkanes
Organoiodine chemistry is the study of the synthesis and properties of organoiodine compounds, or organoiodides, organic compounds that contain one or more carbon–iodine Chemical bond, bonds. They occur widely in organic chemistry, but are relatively rare in nature. The thyroxine hormones are organoiodine compounds that are required for health and the reason for government-mandated iodized salt, iodization of salt. Structure, bonding, general properties Almost all organoiodine compounds feature iodide connected to one carbon center. These are usually classified as derivatives of I−. Some organoiodine compounds feature iodine in higher oxidation states. The C–I bond is the weakest of the carbon–halogen bonds. These bond strengths correlate with the electronegativity of the halogen, decreasing in the order F > Cl > Br > I. This periodic table, periodic order also follows the atomic radius of halogens and the length of the carbon-halogen bond. For example, in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Triiodide
Phosphorus triiodide (PI3) is an inorganic compound with the formula PI3. A red solid, it is too unstable to be stored for long periods of time; it is, nevertheless, commercially available. It is widely used in organic chemistry for converting alcohols to alkyl iodides and also serves as a powerful reducing agent. Properties Although PI3 is a pyramidal molecule, it has only a small molecular dipole because each P-I bond has almost no bond dipole moment. The P-I bond is also weak; PI3 is much less stable than PBr3 and PCl3, with a standard enthalpy of formation for PI3 of only −46 kJ/ mol (solid). The phosphorus atom has an NMR chemical shift of 178 ppm (downfield of H3PO4). Reactions Phosphorus triiodide reacts vigorously with water, producing phosphorous acid (H3PO3) and hydroiodic acid (HI), along with smaller amounts of phosphine and various P-P-containing compounds. Alcohols likewise form alkyl iodides, this providing the main use for PI3. PI3 is also a powerful r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
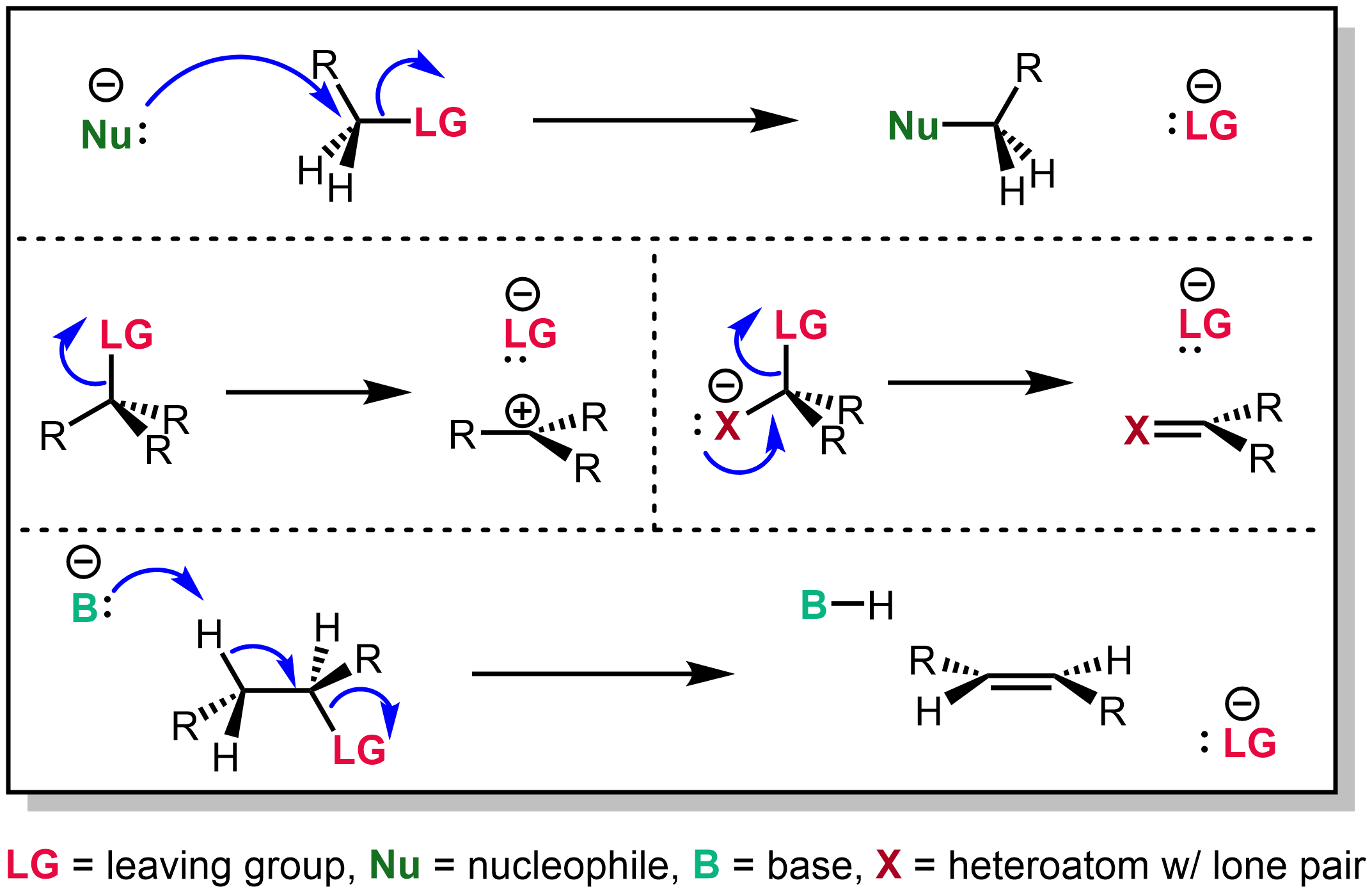
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Iodide Production
Ethyl may refer to: Arts and entertainment *Ethyl Sinclair, a character in the ''Dinosaurs'' television show Science and technology * Ethyl group, an organic chemistry moiety * Ethyl alcohol (or ethanol) * Ethyl Corporation, a fuel additive company ** Tetraethyllead Tetraethyllead (commonly styled tetraethyl lead), abbreviated TEL, is an organolead compound with the formula lead, Pb(ethyl group, C2H5)4. It was widely used as a fuel additive for much of the 20th century, first being mixed with gasoline begi ...-treated gasoline See also * Ethel (other) {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroiodic Acid
Hydroiodic acid (or hydriodic acid) is a colorless liquid. It is an aqueous solution of hydrogen iodide with the chemical formula . It is a strong acid, in which hydrogen iodide is ionized completely in an aqueous solution. Concentrated aqueous solutions of hydrogen iodide are usually 48% to 57% HI by mass. Preparation Reactions Hydroiodic acid reacts with oxygen in air to give iodine: : Like hydrogen halides, hydroiodic acid adds to alkenes to give alkyl iodides. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compounds to anilines. Cativa process The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol. Illicit uses Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrine or pseudoephedrine Pseudoephedrine, sold under the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared artificially, the two most common allotropes being white phosphorus and red phosphorus. With as its only stable isotope, phosphorus has an occurrence in Earth's crust of about 0.1%, generally as phosphate rock. A member of the pnictogen family, phosphorus readily forms a wide variety of organic compound, organic and inorganic compound, inorganic compounds, with as its main oxidation states +5, +3 and −3. The isolation of white phosphorus in 1669 by Hennig Brand marked the scientific community's first discovery since Antiquity of an element. The name phosphorus is a reference to the Phosphorus (morning star), god of the Morning star in Greek mythology, inspired by the faint glow of white phosphorus when exposed to oxygen. This property is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flammable
A combustible material is a material that can burn (i.e., sustain a flame) in air under certain conditions. A material is flammable if it ignites easily at ambient temperatures. In other words, a combustible material ignites with some effort and a flammable material catches fire immediately on exposure to flame. The degree of flammability in air depends largely upon the volatility of the material this is related to its composition-specific vapour pressure, which is temperature dependent. The quantity of vapour produced can be enhanced by increasing the surface area of the material forming a mist or dust. Take wood as an example. Finely divided wood dust can undergo explosive flames and produce a blast wave. A piece of paper (made from pulp) catches on fire quite easily. A heavy oak desk is much harder to ignite, even though the wood fibre is the same in all three materials. Common sense (and indeed scientific consensus until the mid-1700s) would seem to suggest that mate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken or new bonds formed or both. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, usin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |