|
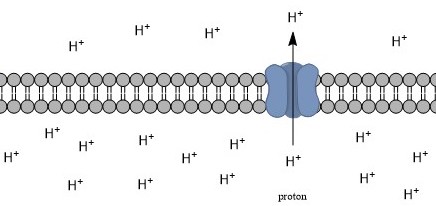
Intracellular PH
Intracellular pH (pHi) is the measure of the acidity or basicity (i.e., pH) of intracellular fluid. The pHi plays a critical role in membrane transport and other intracellular processes. In an environment with the improper pHi, biological cells may have compromised function. Therefore, pHi is closely regulated in order to ensure proper cellular function, controlled cell growth, and normal cellular processes. The mechanisms that regulate pHi are usually considered to be plasma membrane transporters of which two main types exist — those that are dependent and those that are independent of the concentration of bicarbonate (). Physiologically normal intracellular pH is most commonly between 7.0 and 7.4, though there is variability between tissues (e.g., mammalian skeletal muscle tends to have a pHi of 6.8–7.1). There is also pH variation across different organelles, which can span from around 4.5 to 8.0. pHi can be measured in a number of different ways. Homeostasis Intracellul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Membrane PH Gradient
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Biological membranes include cell membranes (outer coverings of cells or organelles that allow passage of certain constituents); nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry (such as chemical plants). This concept of a membrane has been known since the eighteenth century but was used little outside of the laboratory until the end of World War II. Drinking water supplies in Europe had been compromised by The War and membrane filters were used to test for water safety. However, due to the lack of reliability, slow operation, reduced selectivity and elevated costs, membranes were not widely exploited. The first use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
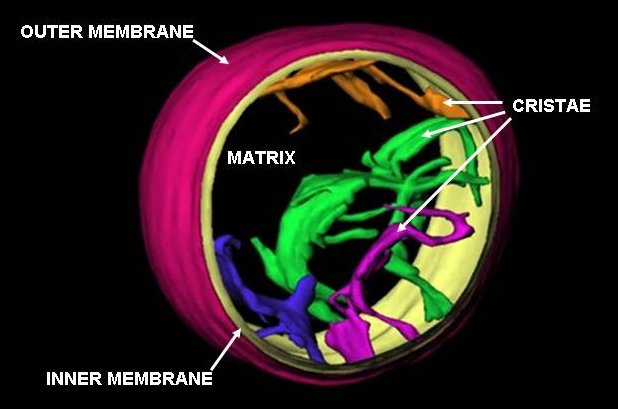
Mitochondria Intermembrane PH
A mitochondrion () is an organelle found in the cell (biology), cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double lipid bilayer, membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'', meaning a thread-like granule, was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase popularized by Philip Siekevitz in a 1957 ''Scientific American'' article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). The multicellular animal ''Henneguya zschokkei, Henneguya salminicola'' is known to have retained mitochondrion-related organelles despite a complete loss of their mitochondrial genome. A large number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
PubMed Identifier
PubMed is an openly accessible, free database which includes primarily the MEDLINE database of references and abstracts on life sciences and biomedical topics. The United States National Library of Medicine (NLM) at the National Institutes of Health maintains the database as part of the Entrez system of information retrieval. From 1971 to 1997, online access to the MEDLINE database was provided via computer and phone lines primarily through institutional facilities, such as university libraries. PubMed, first released in January 1996, ushered in the era of private, free, home- and office-based MEDLINE searching. The PubMed system was offered free to the public starting in June 1997. Content In addition to MEDLINE, PubMed provides access to: * older references from the print version of ''Index Medicus'', back to 1951 and earlier * references to some journals before they were indexed in Index Medicus and MEDLINE, for instance ''Science'', '' BMJ'', and ''Annals of Surgery'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Digital Object Identifier
A digital object identifier (DOI) is a persistent identifier or handle used to uniquely identify various objects, standardized by the International Organization for Standardization (ISO). DOIs are an implementation of the Handle System; they also fit within the URI system ( Uniform Resource Identifier). They are widely used to identify academic, professional, and government information, such as journal articles, research reports, data sets, and official publications. A DOI aims to resolve to its target, the information object to which the DOI refers. This is achieved by binding the DOI to metadata about the object, such as a URL where the object is located. Thus, by being actionable and interoperable, a DOI differs from ISBNs or ISRCs which are identifiers only. The DOI system uses the indecs Content Model to represent metadata. The DOI for a document remains fixed over the lifetime of the document, whereas its location and other metadata may change. Referring to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dissociation (chemistry)
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salt (chemistry), salts, or coordination complex, complexes) separate or split into other things such as atoms, ions, or radical (chemistry), radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegativity, electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination. Dissociation constant For reversible dissociations in a chemical equilibrium :AB A + B the dissociation constant ''K''d is the ratio of dissociated to undissociated compound :K_d = \mathrm where the brackets denote the equilibrium concentrations of the species. Dissociation degree The dissociation degree \alpha is the fraction of original solute molecules that have dissociated. It is usually indicated by the Greek symbol α. More acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
MmHg
A millimetre of mercury is a manometric unit of pressure, formerly defined as the extra pressure generated by a column of mercury one millimetre high. Currently, it is defined as exactly , or approximately 1 torr = atmosphere = pascals.Council Directive 80/181/EEC of 20 December 1979 on the approximation of the laws of the Member States relating to units of measurement and on the repeal of Directive 71/354/EEC of the It is denoted mmHg or mm Hg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
PCO2
''p''CO2, pCO2, or P_\ce is the partial pressure of carbon dioxide (CO2), often used in reference to blood but also used in meteorology, climate science, oceanography, and limnology to describe the fractional pressure of CO2 as a function of its concentration in gas or dissolved phases. The units of ''p''CO2 are mmHg, atm, torr, Pa, or any other standard unit of atmospheric pressure. The ''p''CO2 of Earth's atmosphere has risen from approximately 280 ppm ( parts-per-million) to a mean 2019 value of 409.8 ppm as a result of anthropogenic release of carbon dioxide from fossil fuel burning. This is the highest atmospheric concentration to have existed on Earth for at least the last 800,000 years. Medicine In medicine, the partial pressure of carbon dioxide in arterial blood is called P_ or PaCO2. Measurement of P_ in the systemic circulation indicates the effectiveness of ventilation at the lungs' alveoli, given the diffusing capacity of the gas. It is a good indicator of r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). In respiratory physiology, the partial pressure of a dissolved gas in liquid (such as oxygen in arterial blood) is also defined as the partial pressure of that gas as it would be undissolved in gas phase yet in equilibrium with the liquid. This concept is also known as blood gas tension. In this sense, the diffusion of a gas liquid is said to be driven by differences in partial pressure (not concentration). In chemistry and thermodynamics, this concept is generalized to non-ideal gases and instead called fugacity. The partial pressure of a gas is a measure of its Thermodynamic activity, thermodynamic activity. Gases dissolve, diffuse, and react accor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acidity
An acid is a molecule or ion capable of either donating a proton (i.e. hydrogen cation, H+), known as a Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Arrhenius acids. Brønsted and Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and react with bases and certain metals (like calcium) to form salts. The word ''acid'' is derived from the Latin , meaning 'sour'. An aqueous solution of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |