|
Indoline
Indoline is an aromatic heterocyclic organic compound with the chemical formulation C8H9N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound is based on the indole structure, but the 2-3 bond is saturated. By oxidation/dehydrogenation it can be converted to indole. Indoline can be produced from the reaction of indole, zinc and 85% phosphoric acid Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, .... It was used to make Indocaine. References {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoindoline
Isoindoline is a heterocyclic organic compound with the molecular formula C8H9N. The parent compound has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. The compound's structure is similar to indoline except that the nitrogen atom is in the 2 position instead of the 1 position of the five-membered ring. Isoindoline itself is not commonly encountered, but several derivatives are found in nature and some synthetic derivatives are commercially valuable drugs, e.g. lenalidomide and pazinaclone. Substituted isoindolines 1-Substituted isoindolines and isoindolinones are chiral. Isoindolylcarboxylic acid and 1,3-disubstituted isoindolines are constituents of some pharmaceuticals and natural products. Isoindolines can be prepared by 1,2-addition of a nucleophile onto a bifunctional ε-benzoiminoenoates followed by intramolecular aza-Michael reaction. Another route involves +2cycloaddition of the azomethine ylides (e.g. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
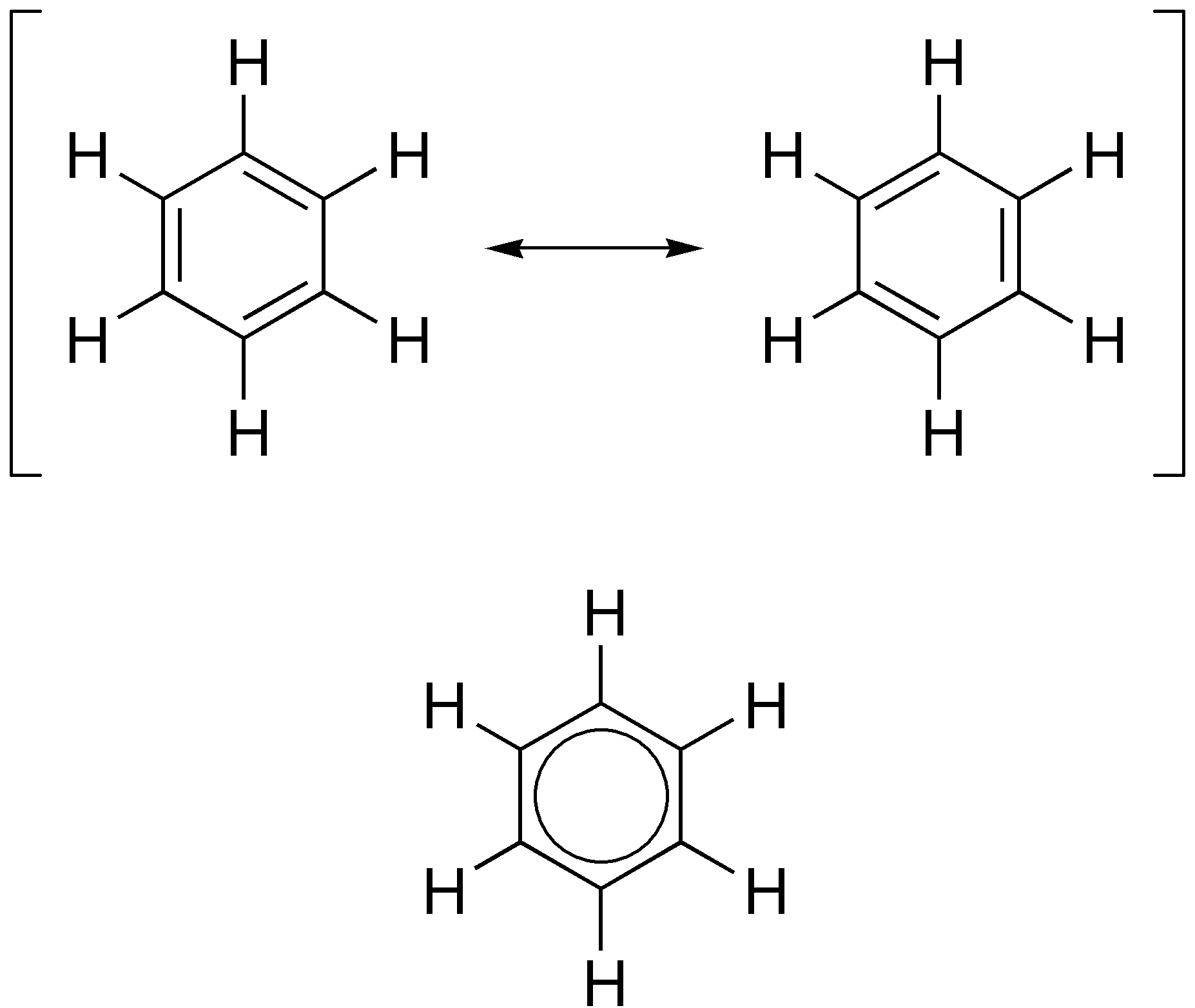
Indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. It has been identified in cannabis. It is the main volatile compound in stinky tofu. When indole is a substituent on a larger molecule, it is called an ''indolyl group'' by systematic nomenclature. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxindole
Oxindole (2-indolone) is an aromatic heterocyclic organic compound with the formula . It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing ring. Oxindole is a modified indoline with a substituted carbonyl at the second position of the 5-member indoline ring. Classified as a cyclic amide, it is a pale yellow solid. Formation and reactions Oxindole is derived in nature from tryptophan, formed by gut bacteria ("normal flora"). It is normally metabolized and detoxified from the body by the liver. In excess, it can cause sedation, muscle weakness, hypotension, and coma. Patients with hepatic encephalopathy Hepatic encephalopathy (HE) is an altered level of consciousness as a result of liver failure. Its onset may be gradual or sudden. Other symptoms may include movement problems, changes in mood, or changes in personality. In the advanced stag ... have been recorded to have elevated serum oxindole levels. Treatmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The earliest use of the term was in an article by August Wilhelm Hofmann in 1855. There is no general relationship between aromaticity as a chemical property and the olfaction, olfactory properties of such compounds. Aromaticity can also be considered a manifestation of cyclic delocalization and of Resonance (chemistry), resonance. This is usually considered to be because electrons are free to cycle around circular arrangements of atoms that are alternately single- and double-covalent bond, bonded to one another. This commonly seen model of aromatic rings, namely the idea that benzene was formed from a six-membered carbon ring with alternating single and double bonds (cyclohexatriene), was developed by Friedrich August Kekulé ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbazole
Carbazole is an aromatic Heterocyclic compound, heterocyclic organic compound. It has a tricyclic structure, consisting of two six-membered benzene rings fused on either side of a five-membered nitrogen-containing ring. The compound's structure is based on the indole structure, but in which a second benzene ring is fused onto the five-membered ring at the 2–3 position of indole (equivalent to the 9a–4a double bond in carbazole, respectively). Carbazole is a constituent of tobacco smoke. History Carl Graebe and Carl Andreas Glaser, Carl Glaser first isolated the compound from coal tar in 1872. Production Few carbazole production methods are economically viable, due to limited demand. During coal tar distillation, carbazole concentrates in the anthracene distillate and must be removed before anthraquinone production; that waste product is the major industrial carbazole source. Polar compounds (e.g., ketones) selectively precipitate it from the anthracene; a more modern techn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. It is a common element in the universe, estimated at Abundance of the chemical elements, seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element chemical bond, bond to form N2, a colourless and odourless diatomic molecule, diatomic gas. N2 forms about 78% of Atmosphere of Earth, Earth's atmosphere, making it the most abundant chemical species in air. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772 and independently by Carl Wilhelm Scheele and Henry Cavendish at about the same time. The name was suggested by French chemist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saturated And Unsaturated Compounds
A saturated compound is a chemical compound (or ion) that resists addition reactions, such as hydrogenation, oxidative addition, and the binding of a Lewis acids and bases, Lewis base. The term is used in many contexts and classes of chemical compounds. Overall, saturated compounds are less reactive than unsaturated compounds. Saturation is derived from the Latin word ''saturare'', meaning 'to fill'.An unsaturated compound is also a chemical compound (or ion) that attracts reduction reactions, such as dehydrogenation,oxidative reduction Organic chemistry Generally distinct types of unsaturated organic compounds are recognized. For hydrocarbons: *alkene (unsaturated) vs alkane (saturated) *alkyne (unsaturated) vs alkane (saturated) *arene (unsaturated) vs cycloalkane (saturated) For organic compounds containing heteroatoms (other than C and H), the list of unsaturated groups is long but some common types are: *carbonyl, e.g. ketones, aldehydes, esters, carboxylic acids (unsatura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |