|
Ibafloxacin
Ibafloxacin (INN) is a fluoroquinolone antibiotic drug formally approved for use in the European Union for veterinary medicine Veterinary medicine is the branch of medicine that deals with the prevention, management, medical diagnosis, diagnosis, and treatment of disease, disorder, and injury in non-human animals. The scope of veterinary medicine is wide, covering all a .... References Flumequines Withdrawn drugs {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoroquinolone
Quinolone antibiotics constitute a large group of broad-spectrum bacteriocidals that share a bicyclic core structure related to the substance 4-quinolone. They are used in human and veterinary medicine to treat bacterial infections, as well as in animal husbandry, specifically poultry production. Quinolone antibiotics are classified into four generations based on their spectrum of activity and chemical modifications. The first-generation quinolones, such as nalidixic acid, primarily target Gram-negative bacteria and are mainly used for urinary tract infections. Second-generation quinolones introduced fluorine atoms into their structure, creating fluoroquinolones, which significantly expanded their antibacterial activity to include some Gram-positive bacteria. Third-generation fluoroquinolones further improved Gram-positive coverage, while fourth-generation fluoroquinolones offer broad-spectrum activity, including anaerobic bacteria. Only quinolone antibiotics in generati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
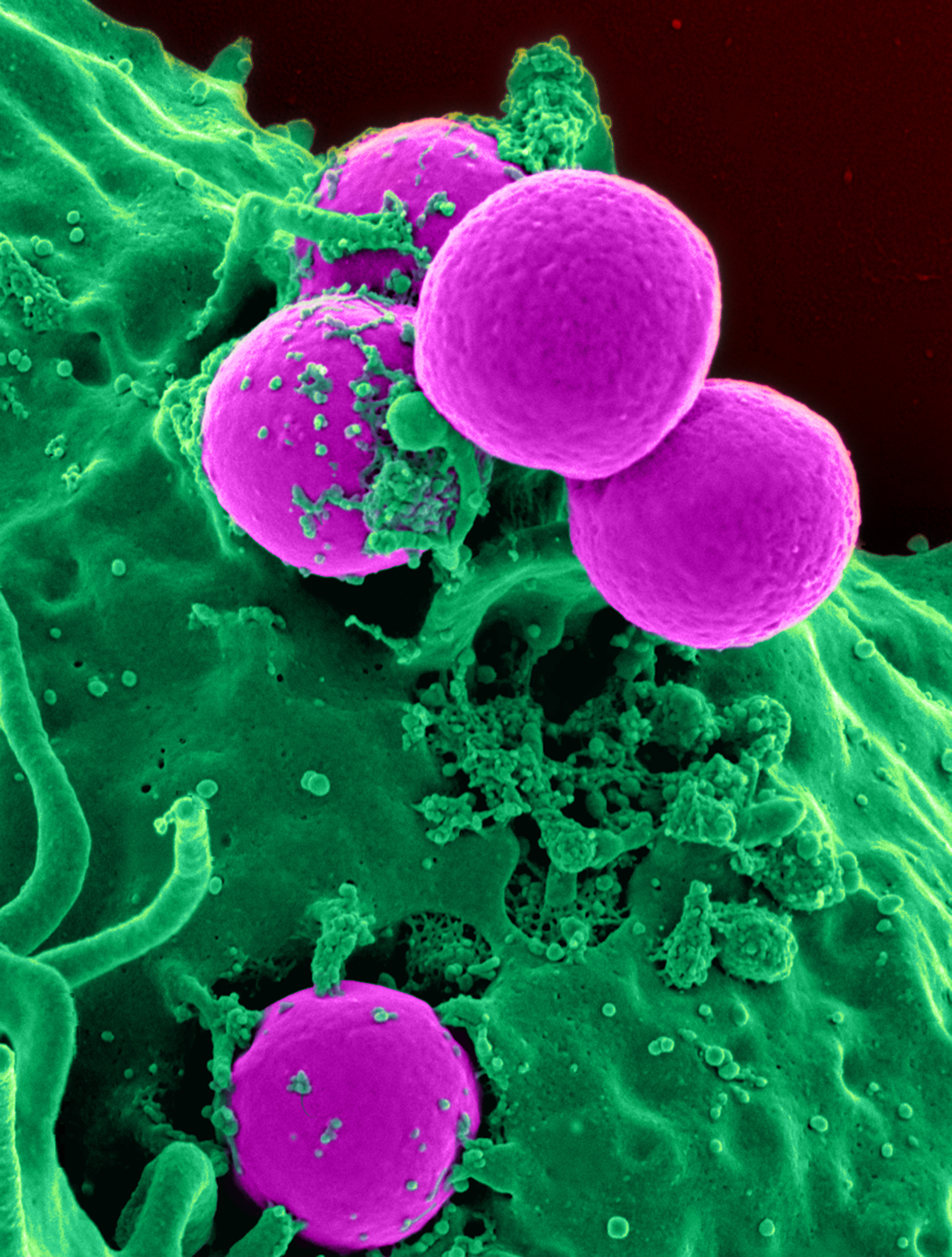
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Veterinary Medicine
Veterinary medicine is the branch of medicine that deals with the prevention, management, medical diagnosis, diagnosis, and treatment of disease, disorder, and injury in non-human animals. The scope of veterinary medicine is wide, covering all animal species, both List of domesticated animals, domesticated and wildlife, wild, with a wide range of conditions that can affect different species. Veterinary medicine is widely practiced, both with and without professional supervision. Professional care is most often led by a veterinarian, veterinary physician (also known as a veterinarian, veterinary surgeon, or "vet"), but also by paraveterinary workers, such as veterinary nurses, veterinary technicians, and veterinary assistants. This can be augmented by other paraprofessionals with specific specialties, such as animal physiotherapy or dentistry, and species-relevant roles such as farriers. Veterinary science helps human health through the monitoring and control of Zoonosis, zoonoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |