|
Hydroxy Radical
The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can accelerate corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of hydrogen peroxide (H2O2) (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds. In organic synthesis, hydroxyl radicals are most commonly generated by photolysis of ''1-Hydroxy-2(1H)-pyri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national List of chemistry societies, chemistry societies, national Academy of Sciences, academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photolysis
Photodissociation, photolysis, photodecomposition, or photofragmentation is a chemical reaction in which molecules of a chemical compound are broken down by absorption of light or photons. It is defined as the interaction of one or more photons with one target molecule that dissociates into two fragments. Here, “light” is broadly defined as radiation spanning the vacuum ultraviolet (VUV), ultraviolet (UV), visible, and infrared (IR) regions of the electromagnetic spectrum. To break covalent bonds, photon energies corresponding to visible, UV, or VUV light are typically required, whereas IR photons may be sufficiently energetic to detach ligands from coordination complexes or to fragment supramolecular complexes. Photolysis in photosynthesis Photolysis is part of the light-dependent reaction, light phase, photochemical phase, or Hill reaction of photosynthesis. The general reaction of photosynthetic photolysis can be given in terms of photons as: :\ce + 2 \text \longri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Macrophage
Macrophages (; abbreviated MPhi, φ, MΦ or MP) are a type of white blood cell of the innate immune system that engulf and digest pathogens, such as cancer cells, microbes, cellular debris and foreign substances, which do not have proteins that are specific to healthy body cells on their surface. This self-protection method can be contrasted with that employed by Natural killer cell, Natural Killer cells. This process of engulfment and digestion is called phagocytosis; it acts to defend the host against infection and injury. Macrophages are found in essentially all tissues, where they patrol for potential pathogens by amoeboid movement. They take various forms (with various names) throughout the body (e.g., histiocytes, Kupffer cells, alveolar macrophages, microglia, and others), but all are part of the mononuclear phagocyte system. Besides phagocytosis, they play a critical role in nonspecific defense (innate immunity) and also help initiate specific defense mechanisms (adapti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immune System
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as Tumor immunology, cancer cells, Parasitic worm, parasitic worms, and also objects such as wood splinters, distinguishing them from the organism's own healthy biological tissue, tissue. Many species have two major subsystems of the immune system. The innate immune system provides a preconfigured response to broad groups of situations and stimuli. The adaptive immune system provides a tailored response to each stimulus by learning to recognize molecules it has previously encountered. Both use humoral immunity, molecules and cell-mediated immunity, cells to perform their functions. Nearly all organisms have some kind of immune system. Bacteria have a rudimentary immune system in the form of enzymes that protect against bacteriophage, viral infections. Other basic immune mechanisms evolved in ancien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxy
In chemistry, peroxides are a group of compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined to each other and to adjacent elements through single covalent bonds, denoted by dashes or lines. The group in a peroxide is often called the peroxide group, though some nomenclature discrepancies exist. This linkage is recognized as a common polyatomic ion, and exists in many molecules. General structure The characteristic structure of any regular peroxide is the oxygen–oxygen covalent single bond, which connects the two main atoms together. In the event that the molecule has no chemical substituents, the peroxide group will have a ��2 net charge. Each oxygen atom has a charge of negative one, as 5 of its valence electrons remain in the outermost orbital shell whilst one is occupied in the covalent bond. Because of the nature of the covalent bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen. The term ''alkyl'' is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cycloalkane by removal of a hydrogen atom from a Ring (chemistry), ring and has the general formula . Typically an alkyl is a part of a larger molecule. In structural formulae, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula . Related concepts Alkylation is the addition of alkyl groups to molecules, often by alkylating agents such as Haloalkane, alkyl halides. Alkylating antineoplastic agents are a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are not simple hydrocarbons. In chemistry, alkyl is a group, a substituent, that is attached to ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatile Organic Compounds
Volatile organic compounds (VOCs) are organic compounds that have a high vapor pressure at room temperature. They are common and exist in a variety of settings and products, not limited to house mold, upholstered furniture, arts and crafts supplies, dry cleaned clothing, and cleaning supplies. VOCs are responsible for the odor of scents and perfumes as well as pollutants. They play an important role in communication between animals and plants, such as attractants for pollinators, protection from predation, and even inter-plant interactions. Some VOCs are dangerous to human health or cause harm to the environment, often despite the odor being perceived as pleasant, such as " new car smell". Anthropogenic VOCs are regulated by law, especially indoors, where concentrations are the highest. Most VOCs are not acutely toxic, but may have long-term chronic health effects. Some VOCs have been used in pharmaceutical settings, while others are the target of administrative controls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbons
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic; their odor is usually faint, and may be similar to that of gasoline or lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are either carbon dioxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorofluorocarbon
Chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) are fully or partly Halogenation, halogenated hydrocarbons that contain carbon (C), hydrogen (H), chlorine (Cl), and fluorine (F). They are produced as volatility (chemistry), volatile derivatives of methane, ethane, and propane. The most common example of a CFC is dichlorodifluoromethane (R-12). R-12, also commonly called Freon, is used as a refrigerant. Many CFCs have been widely used as refrigerants, propellants (in aerosol applications), gaseous fire suppression systems, and solvents. As a result of CFCs contributing to ozone depletion in the upper atmosphere, the manufacture of such compounds has been phased out under the Montreal Protocol, and they are being replaced with other products such as hydrofluorocarbons (HFCs) and hydrofluoroolefins (HFOs) including R-410A, R-134a and 2,3,3,3-Tetrafluoropropene, R-1234yf. Structure, properties and production As in simpler alkanes, carbons in CFCs bond with tetrahe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
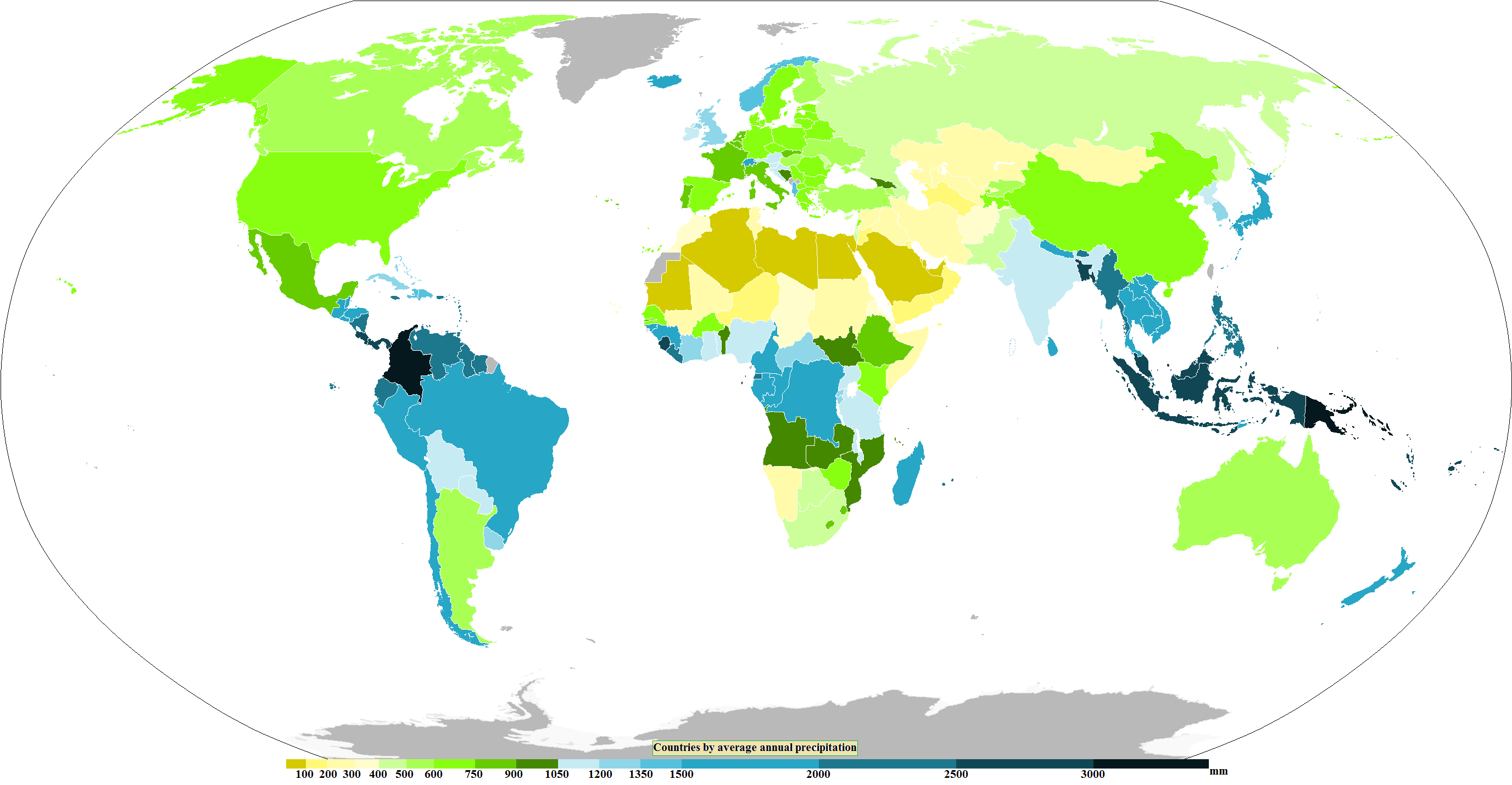
Precipitation
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, rain and snow mixed ("sleet" in Commonwealth usage), snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. (Such a non-precipitating combination is a colloid.) Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are calle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , breaking down in the lower atmosphere to (dioxygen). Ozone is formed from dioxygen by the action of ultraviolet (UV) light and electrical discharges within the Earth's atmosphere. It is present in very low concentrations throughout the atmosphere, with its highest concentration high in the ozone layer of the stratosphere, which absorbs most of the Sun's ultraviolet (UV) radiation. Ozone's odor is reminiscent of chlorine, and detectable by many people at concentrations of as little as in air. Ozone's O3 chemical structure, structure was determined in 1865. The molecule was later proven to have a bent structure and to be weakly diamagnetism, diamagnetic. At standard temperature and pressure, ozone is a pale blue gas that condenses at cryogenic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes it an economically attractive fuel, although capturing and storing it is difficult because it is a gas at standard temperature and pressure. In the Earth's atmosphere methane is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. Methane is an Organic chemistry, organic Organic compound, compound, and among the simplest of organic compounds. Methane is also a hydrocarbon. Naturally occurring methane is found both below ground and under the seafloor and is formed by both geological and biological processes. The largest reservoir of methane is under the seafloor in the form of methane clathrates. When methane reaches the surface and the Atmosphere of Earth, atmosphere, it is known as atmospheric methane. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |