|
Hla-a Antigens
HLA-A is a group of human leukocyte antigens (HLA) that are encoded by the ''HLA-A'' locus, which is located at human chromosome 6p21.3. HLA is a major histocompatibility complex (MHC) antigen specific to humans. HLA-A is one of three major types of human MHC class I transmembrane proteins. The others are HLA-B and HLA-C. The protein is a heterodimer, and is composed of a heavy α chain and smaller β chain. The α chain is encoded by a variant ''HLA-A'' gene, and the β chain (β2-microglobulin) is an invariant β2 microglobulin molecule. The β2 microglobulin protein is encoded by the ''B2M'' gene, which is located at chromosome 15q21.1 in humans. MHC Class I molecules such as HLA-A participate in a process that presents short polypeptides to the immune system. These polypeptides are typically 7–11 amino acids in length and originate from proteins being expressed by the cell. There are two classes of polypeptide that can be presented by an HLA protein: those that are suppos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MHC Class I
MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules (the other being MHC class II) and are found on the cell surface of all nucleated cells in the bodies of vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called ''cytosolic'' or ''endogenous pathway''. In humans, the HLAs corresponding to MHC class I are HLA-A, HLA-B, and HLA-C. Function Class I MHC molecules bind peptides generated mainly from the degradation of cytosolic proteins by the proteasome. The MHC I: peptide complex is then inserted via the endoplasmic re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alleles
An allele is a variant of the sequence of nucleotides at a particular location, or locus, on a DNA molecule. Alleles can differ at a single position through single nucleotide polymorphisms (SNP), but they can also have insertions and deletions of up to several thousand base pairs. Most alleles observed result in little or no change in the function or amount of the gene product(s) they code or regulate for. However, sometimes different alleles can result in different observable phenotypic traits, such as different pigmentation. A notable example of this is Gregor Mendel's discovery that the white and purple flower colors in pea plants were the result of a single gene with two alleles. Nearly all multicellular organisms have two sets of chromosomes at some point in their biological life cycle; that is, they are diploid. For a given locus, if the two chromosomes contain the same allele, they, and the organism, are homozygous with respect to that allele. If the alleles are differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
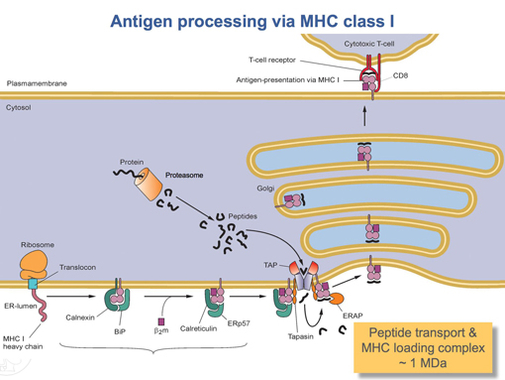
Calnexin
Calnexin (CNX) is a 67kDa integral protein (that appears variously as a 90kDa, 80kDa, or 75kDa band on western blotting depending on the source of the antibody) of the endoplasmic reticulum (ER). It consists of a large (50 kDa) N-terminal calcium- binding lumenal domain, a single transmembrane helix and a short (90 residues), acidic cytoplasmic tail. In humans, calnexin is encoded by the gene ''CANX''. Function Calnexin is a chaperone, characterized by assisting protein folding and quality control, ensuring that only properly folded and assembled proteins proceed further along the secretory pathway. It specifically acts to retain unfolded or unassembled N-linked glycoproteins in the ER. Calnexin binds only those ''N''-glycoproteins that have GlcNAc2Man9Glc1 oligosaccharides. These monoglucosylated oligosaccharides result from the trimming of two glucose residues by the sequential action of two glucosidases, I and II. Glucosidase II can also remove the third and last glu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chaperone Protein
In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis. The first molecular chaperones discovered were a type of assembly chaperones which assist in the assembly of nucleosomes from folded histones and DNA. One major function of molecular chaperones is to prevent the aggregation of misfolded proteins, thus many chaperone proteins are classified as heat shock proteins, as the tendency for protein aggregation is increased by heat stress. The majority of molecular chaperones do not convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermedia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-sheet
The beta sheet (β-sheet, also β-pleated sheet) is a common structural motif, motif of the regular protein secondary structure. Beta sheets consist of beta strands (β-strands) connected laterally by at least two or three backbone chain, backbone hydrogen bonds, forming a generally twisted, pleated sheet. A β-strand is a stretch of peptide, polypeptide chain typically 3 to 10 amino acids long with backbone in an extended conformational isomerism, conformation. The supramolecular association of β-sheets has been implicated in the formation of the Amyloid fibril, fibrils and Amyloid plaques, protein aggregates observed in amyloidosis, Alzheimer's disease and other Proteinopathy, proteinopathies. History The first β-sheet structure was proposed by William Astbury in the 1930s. He proposed the idea of hydrogen bonding between the peptide bonds of parallel or antiparallel extended β-strands. However, Astbury did not have the necessary data on the bond geometry of the amino acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Protein
A transmembrane protein is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them ( beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-pass membrane proteins, or as multipass membrane proteins. Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxic T Cell
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens such as viruses or bacteria, or cells that are damaged in other ways. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigen. An antigen is a molecule capable of stimulating an immune response and is often produced by cancer cells, viruses, bacteria or intracellular signals. Antigens inside a cell are bound to class I MHC molecules, and brought to the surface of the cell by the class I MHC molecule, where they can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecule and the antigen, and the T cell destroys the cell. In order for the TCR to bind to the class I MHC molecule, the former must be accompanied by a glycoprotei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for "little net". It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. There are two types of ER that share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of Cell (biology), cells contain different ratios of the two types of ER dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Signal Peptide
A signal peptide (sometimes referred to as signal sequence, targeting signal, localization signal, localization sequence, transit peptide, leader sequence or leader peptide) is a short peptide (usually 16–30 amino acids long) present at the N-terminus (or occasionally nonclassically at the C-terminus or internally) of most newly synthesized proteins that are destined toward the secretory pathway. These proteins include those that reside either inside certain organelles (the endoplasmic reticulum, Golgi or endosomes), secreted from the cell, or inserted into most cellular membranes. Although most type I membrane-bound proteins have signal peptides, most type II and multi-spanning membrane-bound proteins are targeted to the secretory pathway by their first transmembrane domain, which biochemically resembles a signal sequence except that it is not cleaved. They are a kind of target peptide. Function (translocation) Signal peptides function to prompt a cell to transloc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
History And Naming Of Human Leukocyte Antigens
Human leukocyte antigens (HLA) began as a list of antigens identified as a result of transplant rejection. The antigens were initially identified by categorizing and performing massive statistical analyses on interactions between blood types.Davis, Daniel M. The Compatibility Gene. How Our Bodies Fight Disease, Attract Others, and Define Our Selves. Oxford: Oxford UP, 2014. Print. This process is based upon the principle of serotypes. HLA are not typical antigens, like those found on surface of infectious agents. HLAs are Alloimmunity, ''allo''antigens, they vary from individual to individual as a result of genetic differences. An organ called the thymus is responsible for ensuring that any T-cells that attack self proteins are not allowed to live. In essence, every individual's immune system is tuned to the specific set of HLA and self proteins produced by that individual; where this goes awry is when tissues are transferred to another person. Since individuals almost always have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |