|
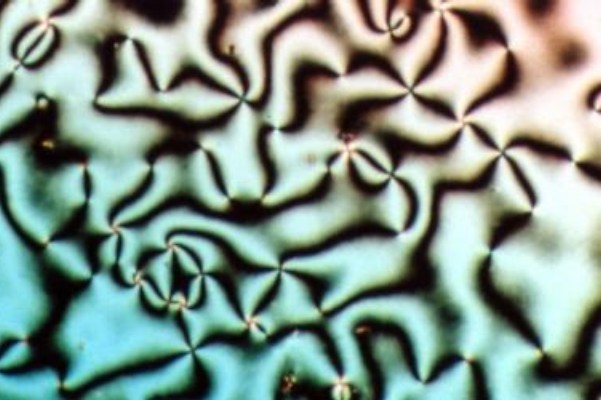
Hexagonal Phase
A hexagonal phase of lyotropic liquid crystal is formed by some amphiphilic molecules when they are mixed with water or another polar solvent. In this phase, the amphiphile molecules are aggregated into cylindrical structures of indefinite length and these cylindrical aggregates are disposed on a hexagonal lattice, giving the phase long-range orientational order. In normal topology hexagonal phases, which are formed by type I amphiphiles, the hydrocarbon chains are contained within the cylindrical aggregates such that the polar-apolar interface has a positive mean curvature. Inverse topology hexagonal phases have water within the cylindrical aggregates and the hydrocarbon chains fill the voids between the hexagonally packed cylinders. Normal topology hexagonal phases are denoted by HI while inverse topology hexagonal phases are denoted by HII. When viewed by polarization microscopy, thin films of both normal and inverse topology hexagonal phases exhibit birefringence, giving ris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Polymorphism
In biophysics and colloidal chemistry, polymorphism is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as " phases". This can be in the form of spheres of lipid molecules (micelles), pairs of layers that face one another ( lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Crystals
Liquid crystal (LC) is a state of matter whose properties are between those of conventional liquids and those of solid crystals. For example, a liquid crystal can flow like a liquid, but its molecules may be oriented in a common direction as in a solid. There are many types of LC phases, which can be distinguished by their optical properties (such as textures). The contrasting textures arise due to molecules within one area of material ("domain") being oriented in the same direction but different areas having different orientations. An LC material may not always be in an LC state of matter (just as water may be ice or water vapour). Liquid crystals can be divided into three main types: thermotropic, lyotropic, and metallotropic. Thermotropic and lyotropic liquid crystals consist mostly of organic molecules, although a few minerals are also known. Thermotropic LCs exhibit a phase transition into the LC phase as temperature changes. Lyotropic LCs exhibit phase transitions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surfactants
Surfactants are chemical compounds that decrease the surface tension or interfacial tension between two liquids, a liquid and a gas, or a liquid and a solid. The word ''surfactant'' is a blend of "surface-active agent", coined in 1950. As they consist of a water-repellent and a water-attracting part, they enable water and oil to mix; they can form foam and facilitate the detachment of dirt. Surfactants are among the most widespread and commercially important chemicals. Private households as well as many industries use them in large quantities as detergents and cleaning agents, but also for example as emulsifiers, wetting agents, foaming agents, antistatic additives, or dispersants. Surfactants occur naturally in traditional plant-based detergents, e.g. horse chestnuts or soap nuts; they can also be found in the secretions of some caterpillars. Today one of the most commonly used anionic surfactants, linear alkylbenzene sulfates (LAS), are produced from petroleum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Micelle
A micelle () or micella () ( or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloidal system). A typical micelle in water forms an aggregate, with the hydrophilic "head" regions in contact with surrounding solvent, sequestering the hydrophobic single-tail regions in the micelle centre. This phase is caused by the packing behavior of single-tail lipids in a bilayer. The difficulty in filling the volume of the interior of a bilayer, while accommodating the area per head group forced on the molecule by the hydration of the lipid head group, leads to the formation of the micelle. This type of micelle is known as a normal-phase micelle (or oil-in-water micelle). Inverse micelles have the head groups at the centre with the tails extending out (or water-in-oil micelle). Micelles are approximately spherical in shape. Other shapes, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Polymorphism
In biophysics and colloidal chemistry, polymorphism is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as " phases". This can be in the form of spheres of lipid molecules (micelles), pairs of layers that face one another ( lamellar phase, observed in biological systems as a lipid bilayer), a tubular arrangement (hexagonal), or various cubic phases (Fdm, Imm, Iam, Pnm, and Pmm being those discovered so far). More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases. It forms an important part of current academic research in the fields of membrane biophysics (polymorphism), biochemistry (biological impact) and organic chemistry (synthesis). Determination of the topology of a lipid system is possible by a number of methods, the most reliable of which is x-ray diffraction. This uses a beam of x-rays that are scattered by the sample, giving a diffraction pattern as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lamellar Phase
Lamellar phase refers generally to packing of polar-headed, long chain, nonpolar-tailed molecules (amphiphiles) in an environment of bulk polar liquid, as sheets of bilayers separated by bulk liquid. In biophysics, polar lipids (mostly, phospholipids, and rarely, glycolipids) pack as a liquid crystalline bilayer, with hydrophobic fatty acyl long chains directed inwardly and polar headgroups of lipids aligned on the outside in contact with water, as a 2-dimensional flat sheet surface. Under transmission electron microscopy (TEM), after staining with polar headgroup reactive chemical osmium tetroxide, lamellar lipid phase appears as two thin parallel dark staining lines/sheets, constituted by aligned polar headgroups of lipids. 'Sandwiched' between these two parallel lines, there exists one thicker line/sheet of non-staining closely packed layer of long lipid fatty acyl chains. This TEM-appearance became famous as Robertson's unit membrane - the basis of all biological membran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Negative Staining
In microscopy, negative staining is an established method, often used in diagnostic microscopy, for contrasting a thin biological specimen, specimen with an optics, optically opacity (optics), opaque fluid. In this technique, the background is staining, stained, leaving the actual specimen untouched, and thus visible. This contrasts with positive staining, in which the actual specimen is stained. Bright field microscopy For bright-field microscopy, negative staining is typically performed using a black ink fluid such as nigrosin and India ink. The specimen, such as a wet bacterial culture spread on a glass slide, is mixed with the negative stain and allowed to dry. When viewed with the microscope the bacterial cells, and perhaps their spores, appear light against the dark surrounding background. An alternative method has been developed using an ordinary waterproof marking pen to deliver the negative stain. Transmission electron microscopy In the case of transmission electron micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipid Bilayer Phase Behavior
In colloidal chemistry, one property of a lipid bilayer is the relative mobility (fluidity) of the individual lipid molecules and how this mobility changes with temperature. This response is known as the phase behavior of the bilayer. Broadly, at a given temperature a lipid bilayer can exist in either a liquid or a solid phase. The solid phase is commonly referred to as a “gel” phase. All lipids have a characteristic temperature at which they undergo a Phase transition, transition (melting, melt) from the gel to liquid phase. In both phases the lipid molecules are constrained to the two dimensional plane of the membrane, but in liquid phase bilayers the molecules diffuse freely within this plane. Thus, in a liquid bilayer a given lipid will rapidly exchange locations with its neighbor millions of times a second and will, through the process of a random walk, migrate over long distances.H. C. Berg, "Random Walks in Biology". Extended Paperback Ed. ed. 1993, Princeton, NJ: Princet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lyotropic Liquid Crystal
Lyotropic liquid crystals result when Amphiphile, amphiphiles, which are both hydrophobic and hydrophilic, dissolve into a Solution (chemistry), solution that behaves both like a liquid and a solid crystal. This liquid crystalline mesophase includes everyday mixtures like soap and water. The term ' comes . Historically, the term was used to describe the common behavior of materials composed of amphiphilic molecules upon the addition of a solvent. Such molecules comprise a hydrophilic (literally 'water-loving') head-Functional group, group (which may be ionic or non-ionic) attached to a hydrophobic ('water-hating') group. The micro-phase segregation of two incompatible components on a nanometer scale results in different type of solvent-induced extended anisotropic arrangement, depending on the volume balances between the hydrophilic part and hydrophobic part. In turn, they generate the long-range order of the phases, with the solvent molecules filling the space around the compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Birefringence
Birefringence, also called double refraction, is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are described as birefringent or birefractive. The birefringence is often quantified as the maximum difference between refractive indices exhibited by the material. Crystals with non-cubic crystal structures are often birefringent, as are plastics under mechanical stress. Birefringence is responsible for the phenomenon of double refraction whereby a ray of light, when incident upon a birefringent material, is split by polarization into two rays taking slightly different paths. This effect was first described by Danish scientist Rasmus Bartholin in 1669, who observed it in Iceland spar (calcite) crystals which have one of the strongest birefringences. In the 19th century Augustin-Jean Fresnel described the phenomenon in terms of polarization, understanding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarization Microscopy
Polarized light microscopy can mean any of a number of optical microscopy techniques involving polarized light. Simple techniques include illumination of the sample with polarized light. Directly transmitted light can, optionally, be blocked with a polariser oriented at 90 degrees to the illumination. More complex microscopy techniques which take advantage of polarized light include differential interference contrast microscopy and interference reflection microscopy. Scientists will often use a device called a polarizing plate to convert natural light into polarized light. These illumination techniques are most commonly used on birefringent samples where the polarized light interacts strongly with the sample and so generating contrast with the background. Polarized light microscopy is used extensively in optical mineralogy. Although the invention of the polarizing microscope is typically attributed to David Brewster around 1815, Brewster clearly acknowledges the priority of Henr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |