|
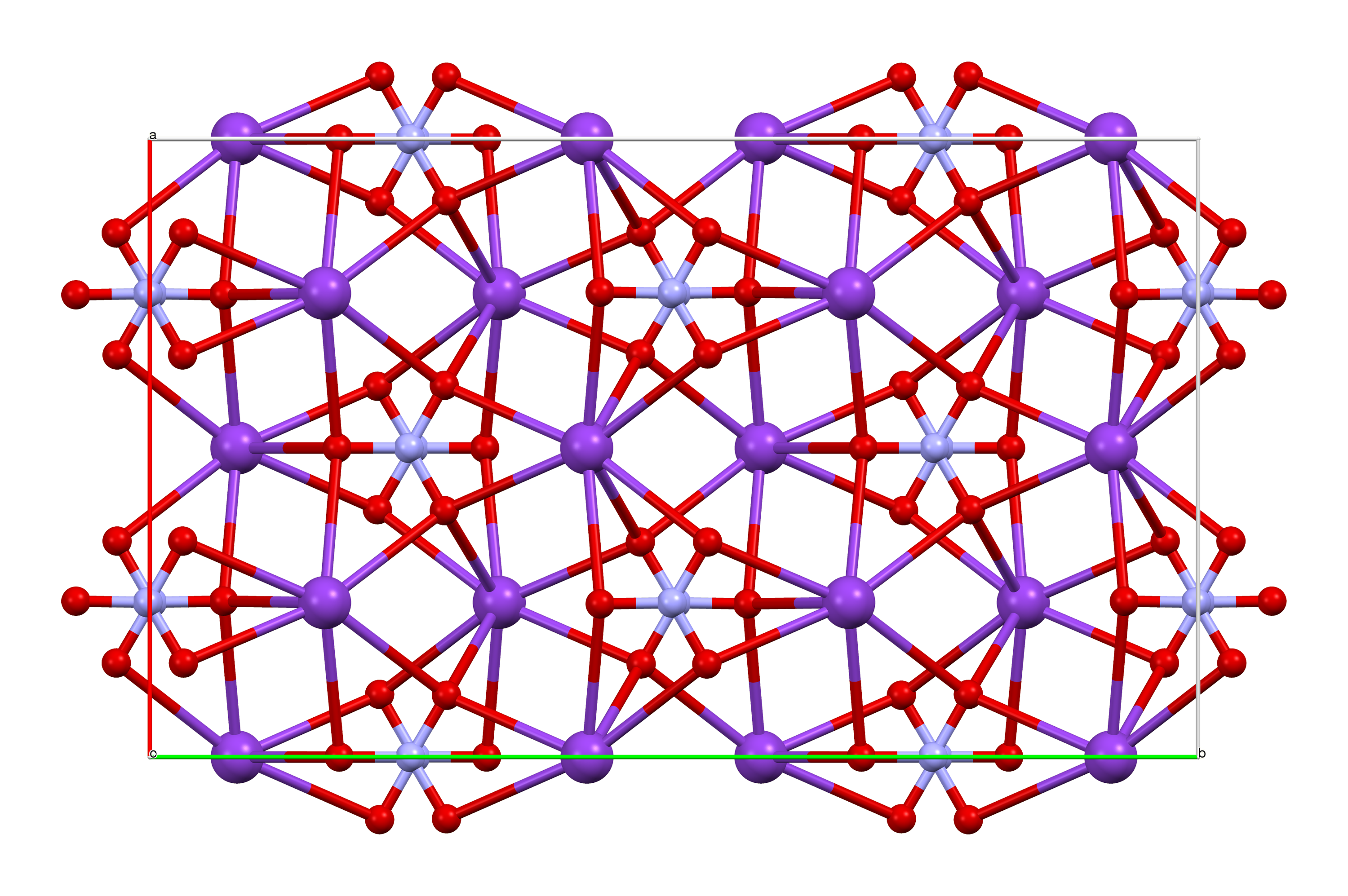
Hexachlorobenzene
Hexachlorobenzene, or perchlorobenzene, is an aryl chloride and a six-substituted chlorobenzene with the molecular formula C6Cl6. It is a fungicide formerly used as a seed treatment, especially on wheat to control the fungal disease bunt. Its use has been banned globally under the Stockholm Convention on Persistent Organic Pollutants. Physical and chemical properties Hexachlorobenzene is a stable, white, crystalline chlorinated hydrocarbon. It is sparingly soluble in organic solvents such as benzene, diethyl ether and alcohol, but practically insoluble in water with no reaction. It has a flash point of 468 °F and it is stable under normal temperatures and pressures. It is combustible but it does not ignite readily. When heated to decomposition, hexachlorobenzene emits highly toxic fumes of hydrochloric acid, other chlorinated compounds (such as phosgene), carbon monoxide, and carbon dioxide. History Hexachlorobenzene was first known as "Julin's chloride of carbon" as it w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hugo Müller
Hugo Müller (29 July 1833 – 23 May 1915) was an Anglo-German analytical chemist, botanist and industrialist. He was a Fellow of the Royal Society. He is known for being the first person to synthesize hexachlorobenzene. Early life Hugo Müller was born on 29 July, 1833, in Tirschenreuth, Germany. He studied chemistry in Leipzig University. He was a student of Friedrich Wöhler at the University of Göttingen, where he earned his PhD. He was also assistant to Justus von Liebig at the University of Munich. Career Müller moved to United Kingdom in 1855 to work with Warren De la Rue as recommended by Liebig. De la Rue and Müller developed a chloride of silver battery. He earned a Legum Doctor degree from the University of St Andrews and a Doctor of Science degree from the University of Manchester. He also worked with John Stenhouse. Müller discovered that iodine could be used as a catalyst in chlorination. In 1864, Müller discovered how to transform mono-functional c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethylene
Tetrachloroethylene, also known as perchloroethylene or under the systematic name tetrachloroethene, and abbreviations such as perc (or PERC), and PCE, is a chlorocarbon with the formula . It is a non-flammable, stable, colorless and heavy liquid widely used for dry cleaning of fabrics and occasionally as a highly effective automotive brake cleaner. It has a mildly sweet, sharp odor, detectable by most people at a concentration of 50 ppm. Tetrachloroethylene is regarded as a toxic substance, a human health hazard, and an environmental hazard. In 2020, the United States Environmental Protection Agency stated that "tetrachloroethylene exposure may harm the nervous system, liver, kidneys, and reproductive system, and may be harmful to unborn children", and reported that numerous toxicology agencies regard it as a carcinogen. History and production French chemist Henri Victor Regnault first synthesized tetrachloroethylene in 1839 by thermal decomposition of hexachloroethane f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobenzene
Hexafluorobenzene, HFB or perfluorobenzene is an organofluorine compound with the chemical formula . In this derivative of benzene, all hydrogen atoms have been replaced by fluorine atoms. The technical uses of the compound are limited, although it has some specialized uses in the laboratory owing to distinctive spectroscopic properties. Geometry of the aromatic ring Hexafluorobenzene stands somewhat aside in the perhalogenbenzenes. If a perhalogenated benzene ring were to remain planar, then geometric constraints would force adjacent halogens closer than their associated nonbonding radius. Consequently the benzene ring buckles, reducing ''p''-orbital overlap and aromaticity to avoid the steric clash. Perfluorobenzene is an exception: as shown in the following table, two fluorines are small enough to avoid collision, retaining planarity and full aromaticity. Synthesis The direct synthesis of hexafluorobenzene from benzene and fluorine has not been useful. Instead it is pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stockholm Convention On Persistent Organic Pollutants
Stockholm Convention on Persistent Organic Pollutants is an international environmental treaty, signed on 22 May 2001 in Stockholm and effective from 17 May 2004, that aims to eliminate or restrict the production and use of persistent organic pollutants (POPs). History In 1995, the Governing Council of the United Nations Environment Programme (UNEP) called for global action to be taken on POPs, which it defined as "chemical substances that persist in the environment, bio-accumulate through the food web, and pose a risk of causing adverse effects to human health and the environment". Following this, the Intergovernmental Forum on Chemical Safety (IFCS) and the International Programme on Chemical Safety (IPCS) prepared an assessment of the 12 worst offenders, known as the ''dirty dozen''. The INC met five times between June 1998 and December 2000 to elaborate the convention, and delegates adopted the Stockholm Convention on POPs at the Conference of the Plenipotentiaries ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula . It is an Alcohol (chemistry), alcohol, with its formula also written as , or EtOH, where Et is the pseudoelement symbol for ethyl group, ethyl. Ethanol is a Volatility (chemistry), volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. Historically it was used as a general anesthetic, and has modern medical applications as an antiseptic, disinfectant, solvent for some medications, and antidote for methanol poisoning and ethylene glycol poisoning. It is used as a chemical solvent and in the Chemical synthesis, synthesis of orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Henry Bassett
Henry may refer to: People and fictional characters * Henry (given name), including lists of people and fictional characters * Henry (surname) * Henry, a stage name of François-Louis Henry (1786–1855), French baritone Arts and entertainment * ''Henry'' (2011 film), a Canadian short film * ''Henry'' (2015 film), a virtual reality film * '' Henry: Portrait of a Serial Killer'', a 1986 American crime film * ''Henry'' (comics), an American comic strip created in 1932 by Carl Anderson * "Henry", a song by New Riders of the Purple Sage Places Antarctica * Henry Bay, Wilkes Land Australia *Henry River (New South Wales) *Henry River (Western Australia) Canada * Henry Lake (Vancouver Island), British Columbia * Henry Lake (Halifax County), Nova Scotia * Henry Lake (District of Chester), Nova Scotia New Zealand * Lake Henry (New Zealand) * Henry River (New Zealand) United States * Henry, Illinois * Henry, Indiana * Henry, Nebraska * Henry, South Dakota * Henry County (disambigu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Faraday
Michael Faraday (; 22 September 1791 – 25 August 1867) was an English chemist and physicist who contributed to the study of electrochemistry and electromagnetism. His main discoveries include the principles underlying electromagnetic induction, diamagnetism, and electrolysis. Although Faraday received little formal education, as a self-made man, he was one of the most influential scientists in history. It was by his research on the magnetic field around a Electrical conductor, conductor carrying a direct current that Faraday established the concept of the electromagnetic field in physics. Faraday also established that magnetism could Faraday effect, affect rays of light and that there was an underlying relationship between the two phenomena. the 1911 ''Encyclopædia Britannica''. He similarly discovered the principles of electromagnetic induction, diamagnetism, and the Faraday's laws of electrolysis, laws of electrolysis. His inventions of electric motor, electromagnetic rotar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexachloroethane
Hexachloroethane (perchloroethane) is an organochlorine compound with the chemical formula . Its structure is . It is a white or colorless solid at room temperature with a camphor-like odor. It has been used by the military in smoke compositions, such as base-eject smoke munitions ( smoke grenades). Hexachloroethane was discovered along with carbon tetrachloride by Michael Faraday in 1820. Faraday obtained it by chlorinating ethylene. Manufacture Chlorination of tetrachloroethylene at 100–140 °C with the presence of iron(III) chloride is the most commonly used commercial production method, however several other methods exist. A high purity form can be produced in a small scale by reacting chlorine together with barium carbide. In September 1997, it was reported as no longer being produced in the United States for commercial distribution, but was produced as a by-product of industrial chlorination process. : Applications Hexachloroethane has been used in the formulation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Richard Phillips (chemist)
Richard Phillips FRS FRSE FCS FGS (21 November 1778 – 11 May 1851), was a distinguished British chemist and became a fellow of the Royal Society in 1822. Early life Born in Lombard Street, London on 21 November 1778, he was the son of James Phillips (1745–1799), a printer and bookseller and his wife, Mary. He trained in London, receiving his education as a chemist under George Fordyce and William Allen. In 1796, he and his brother William, together with William Allen and Luke Howard, took part in forming the Askesian Society. He then went on to be founder member of the Geological Society after the Askesian Society disbanded in 1807. Academic work A talented chemist, he lectured in chemistry at Royal London Hospital from 1817 and was employed as a professor at Royal Military College in 1818. His proficiency was recognised when he was elected as a fellow of the Royal Society of Edinburgh in 1819, having been proposed by Thomas Allan, Leonard Horner and Sir David Br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saltpetre
Potassium nitrate is a chemical compound with a sharp, salty, bitter taste and the chemical formula . It is a potassium salt of nitric acid. This salt consists of potassium cations and nitrate anions , and is therefore an alkali metal nitrate. It occurs in nature as a mineral, niter (or ''nitre'' outside the United States). It is a source of nitrogen, and nitrogen was named after niter. Potassium nitrate is one of several nitrogen-containing compounds collectively referred to as saltpetre (or saltpeter in the United States). Major uses of potassium nitrate are in fertilizers, tree stump removal, rocket propellants and fireworks. It is one of the major constituents of traditional gunpowder (black powder). In processed meats, potassium nitrate reacts with hemoglobin and myoglobin generating a red color. Etymology Nitre, or potassium nitrate, because of its early and global use and production, has many names. As for nitrate, Egyptian and Hebrew words for it had the consonants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leopold Gmelin
Leopold Gmelin (2 August 1788 – 13 April 1853) was a German chemist. Gmelin was a professor at the University of Heidelberg. He worked on the Potassium ferricyanide, red prussiate and created Gmelin's test, and wrote his ''Handbook of Chemistry'', which over successive editions became a standard reference work still in use. Life Gmelin was a son of the physician, botanist and chemist Johann Friedrich Gmelin and his wife Rosine Schott. Due to his family he early came in contact with medicine and the natural sciences, in 1804 he attended the chemical lectures of his father. In the same year Gmelin moved to Tübingen to work in the family pharmacy, he also studied at the University of Tübingen among other relatives including Ferdinand Gottlieb Gmelin (a cousin) and Carl Friedrich Kielmeyer (husband of a cousin). Supported by Kielmeyer, Gmelin moved to the University of Göttingen in 1805 and later he worked as assistant in the laboratory of Friedrich Stromeyer, by whom he suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cast Iron
Cast iron is a class of iron–carbon alloys with a carbon content of more than 2% and silicon content around 1–3%. Its usefulness derives from its relatively low melting temperature. The alloying elements determine the form in which its carbon appears: Cast iron#White cast iron, white cast iron has its carbon combined into an iron carbide named cementite, which is very hard, but brittle, as it allows cracks to pass straight through; Grey iron, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and Ductile iron, ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing. Carbon (C), ranging from 1.8 to 4 wt%, and silicon (Si), 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as steel. Cast iron tends to be brittle, except for malleable iron, malleable cast irons. With its relatively low melting point, g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |