|
Hexabenzocoronene
Hexa-peri-hexabenzocoronene (HBC) is a polycyclic aromatic hydrocarbon with the molecular formula C42H18. It consists of a central coronene molecule, with an additional benzene ring fused between each adjacent pair of rings around the periphery. It is sometimes simply called hexabenzocoronene, however, there are other chemicals that share this less-specific name, such as hexa-cata-hexabenzocoronene. Hexa-peri-hexabenzocoronene has been imaged by atomic force microscopy (AFM) providing the first example of a molecule in which differences in bond order and bond lengths of the individual bonds can be distinguished by a measurement in direct space. Supramolecular structures Various hexabenzocoronenes have been investigated in supramolecular electronics. They are known to self-assemble into a columnar phase. One derivative in particular forms carbon nanotubes with interesting electrical properties. The columnar phase in this compound further organises itself into sheets, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Aromatic Hydrocarbon
A Polycyclic aromatic hydrocarbon (PAH) is any member of a class of organic compounds that is composed of multiple fused aromatic rings. Most are produced by the incomplete combustion of organic matter— by engine exhaust fumes, tobacco, incinerators, in roasted meats and cereals, or when biomass burns at lower temperatures as in forest fires. The simplest representative is naphthalene, having two aromatic rings, and the three-ring compounds anthracene and phenanthrene. PAHs are uncharged, non-polar and planar. Many are colorless. Many of them are also found in fossil fuel deposits such as coal and in petroleum. Exposure to PAHs can lead to different types of cancer, to fetal development complications, and to cardiovascular issues. Polycyclic aromatic hydrocarbons are discussed as possible starting materials for abiotic syntheses of materials required by the earliest forms of life. Nomenclature and structure The terms polyaromatic hydrocarbon, or polynuclear aromatic hydro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexa-cata-hexabenzocoronene
Hexa-''cata''-hexabenzocoronene (hexabenzo 'a'',''d'',''g'',''j'',''m'',''p''oronene) is a polycyclic aromatic hydrocarbon with the molecular formula C48H24. It consists of a central coronene molecule, with an additional benzene ring fused onto each ring around the periphery. Hexa-''cata''-hexabenzocoronene has a contorted structure due to steric crowding among the benzene rings around the edge, analogous to the situation in benzo 'c''henanthrene. See also * Hexabenzocoronene Hexa-peri-hexabenzocoronene (HBC) is a polycyclic aromatic hydrocarbon with the molecular formula C42H18. It consists of a central coronene molecule, with an additional benzene ring fused between each adjacent pair of rings around the periphery. ... References External links * Polycyclic aromatic hydrocarbons {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic-force Microscopy
Atomic force microscopy (AFM) or scanning force microscopy (SFM) is a very-high-resolution type of scanning probe microscopy (SPM), with demonstrated resolution on the order of fractions of a nanometer, more than 1000 times better than the optical diffraction limit. Overview Atomic force microscopy (AFM) gathers information by "feeling" or "touching" the surface with a mechanical probe. Piezoelectric elements that facilitate tiny but accurate and precise movements on (electronic) command enable precise scanning. Despite the name, the Atomic Force Microscope does not use the nuclear force. Abilities and spatial resolution The AFM has three major abilities: force measurement, topographic imaging, and manipulation. In force measurement, AFMs can be used to measure the forces between the probe and the sample as a function of their mutual separation. This can be applied to perform force spectroscopy, to measure the mechanical properties of the sample, such as the sample's Young' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Scanning Probe Microscopy
Scanning probe microscopy (SPM) is a branch of microscopy that forms images of surfaces using a physical probe that scans the specimen. SPM was founded in 1981, with the invention of the scanning tunneling microscope, an instrument for imaging surfaces at the atomic level. The first successful scanning tunneling microscope experiment was done by Gerd Binnig and Heinrich Rohrer. The key to their success was using a feedback loop to regulate gap distance between the sample and the probe. Many scanning probe microscopes can image several interactions simultaneously. The manner of using these interactions to obtain an image is generally called a mode. The resolution varies somewhat from technique to technique, but some probe techniques reach a rather impressive atomic resolution. This is largely because piezoelectricity, piezoelectric actuators can execute motions with a precision and accuracy at the atomic level or better on electronic command. This family of techniques can be cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrocyclic Reaction
In organic chemistry, an electrocyclic reaction is a type of pericyclic reaction, pericyclic, rearrangement reaction, rearrangement reaction where the net result is one pi bond being converted into one sigma bond or vice versa. These reactions are usually categorized by the following criteria: * Reactions can be either photochemistry, photochemical or thermal. * Reactions can be either ring-opening or ring-closing (electrocyclization). * Depending on the type of reaction (Organic photochemistry, photochemical or thermal) and the number of pi electrons, the reaction can happen through either a conrotatory and disrotatory, conrotatory or disrotatory mechanism. * The type of rotation determines whether the cis–trans isomerism, cis or trans isomer of the product will be formed. Classical examples The Nazarov cyclization reaction is a named electrocyclic reaction converting divinylketones to cyclopentenones. A classic example is the thermal ring-opening reaction of 3,4-dimethylcyclo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexaphenylbenzene
Hexaphenylbenzene is an aromatic compound composed of a benzene ring substituted with six phenyl rings. It is a colorless solid. The compound is the parent member of a wider class of hexaarylbenzenes, which are mainly of theoretical interest. Preparation It is prepared by heating tetraphenylcyclopentadienone and diphenylacetylene in benzophenone or other high-temperature solvent. The reaction proceeds via a Diels–Alder reaction to give the hexaphenyldienone, which then eliminates carbon monoxide. : Together with 1,2,3,4-tetraphenylnaphthalene, hexaphenylbenzene forms by the chromium-catalyzed oligomerization of diphenylacetylene. It may also be prepared by the dicobalt octacarbonyl-catalyzed alkyne trimerisation An alkyne trimerisation is a +2+2nbsp; cycloaddition reaction in which three alkyne units () react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthes ... of diphenylacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Monoxide
Carbon monoxide (chemical formula CO) is a poisonous, flammable gas that is colorless, odorless, tasteless, and slightly less dense than air. Carbon monoxide consists of one carbon atom and one oxygen atom connected by a triple bond. It is the simplest oxocarbon, carbon oxide. In coordination complexes, the carbon monoxide ligand is called ''metal carbonyl, carbonyl''. It is a key ingredient in many processes in industrial chemistry. The most common source of carbon monoxide is the partial combustion of carbon-containing compounds. Numerous environmental and biological sources generate carbon monoxide. In industry, carbon monoxide is important in the production of many compounds, including drugs, fragrances, and fuels. Indoors CO is one of the most acutely toxic contaminants affecting indoor air quality. CO may be emitted from tobacco smoke and generated from malfunctioning fuel-burning stoves (wood, kerosene, natural gas, propane) and fuel-burning heating systems (wood, oil, n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
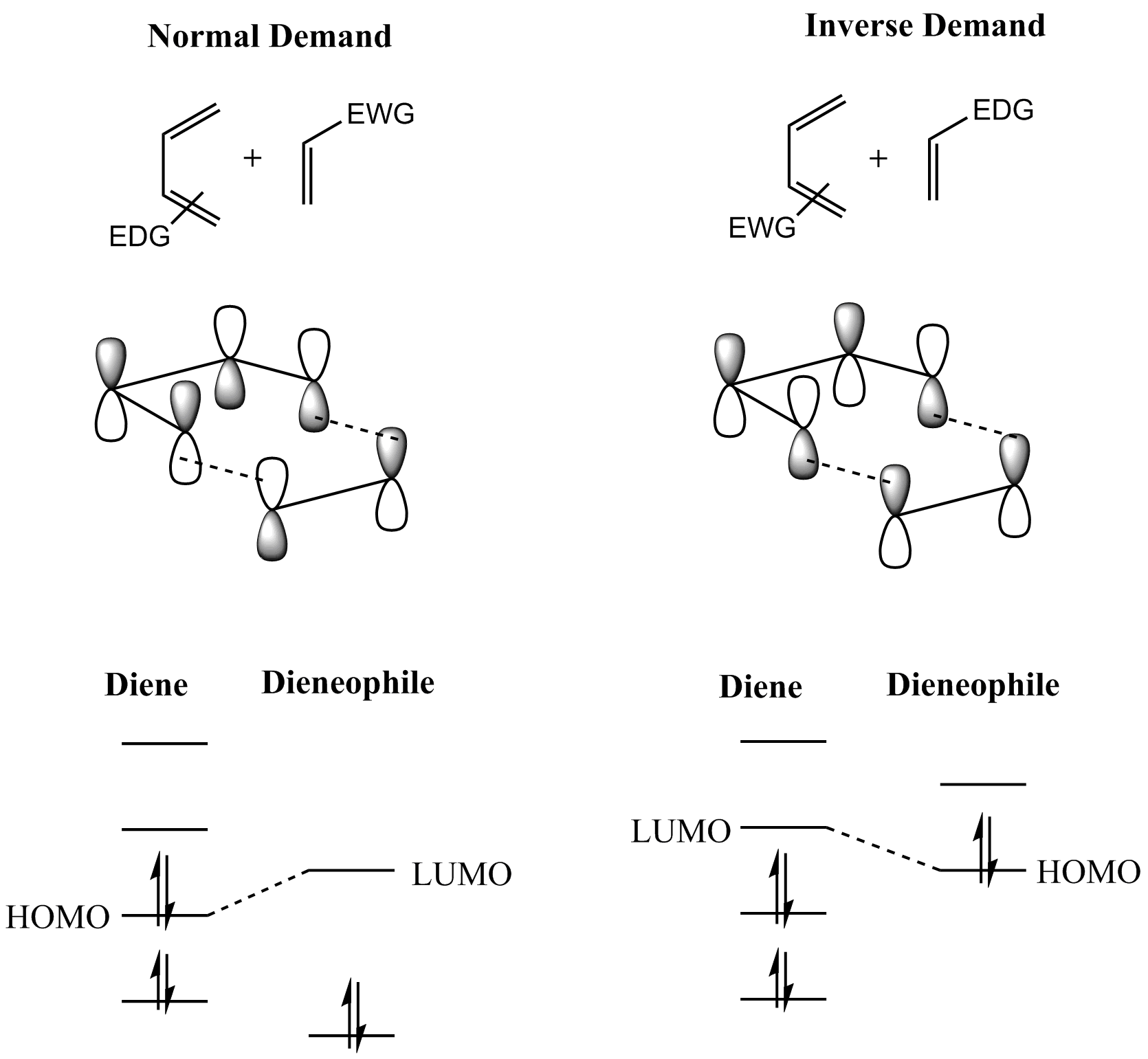
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienone
Cyclopentadienone is an organic compound with molecular formula C5H4O. The parent cyclopentadienone is rarely encountered, because it rapidly dimerizes. Many substituted derivatives are known, notably tetraphenylcyclopentadienone. Such compounds are used as ligands in organometallic chemistry Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so .... left, The Knölker complex, derived from a substituted cyclopentadienone, is a catalyst for transfer hydrogenation. Preparation Cyclopentadienone can be generated by the photolysis or pyrolysis of various substances (e.g. 1,2-benzoquinone), and then isolated in an matrix isolation, argon matrix at . It dimerizes readily upon thawing the matrix at . See also * Dienone References {{Cyclopentadienide complexes Ketones Fully conjugat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzil
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula ( C6H5 CO)2, generally abbreviated ( PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Weinheim. Structure The compound's most noteworthy structural feature is the long carbon-carbon bond of 1.54 Å, which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a dihedral angle of 117°. In less hindered analogues (glyoxal, biacetyl, oxalic acid derivatives), the (RCO)2 group adopts a planar, anti-conformation. Applications Most benzil can be used as a photoinitiator in the free-radical curing of polymer networks. It absorbs ultraviolet radiation at a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzyl Ketone
Dibenzyl ketone, or 1,3-diphenylacetone, is an organic compound composed of two benzyl groups attached to a central carbonyl group. This results in the central carbonyl carbon atom being electrophilic and the two adjacent carbon atoms slightly nucleophilic. For this reason, dibenzyl ketone is frequently used in an aldol condensation reaction with benzil (a dicarbonyl) and base to create tetraphenylcyclopentadienone. Vera Bogdanovskaia is credited with the classification of dibenzyl ketone. Preparation Dibenzyl ketone is prepared by ketonic decarboxylation of phenylacetic acid. One method is where phenylacetic acid is reacted with acetic anhydride and anhydrous potassium acetate and refluxed for two hours at 140−150 °C. The mixture is distilled slowly so that the distillate is mostly acetic acid. Carbon dioxide Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |