|
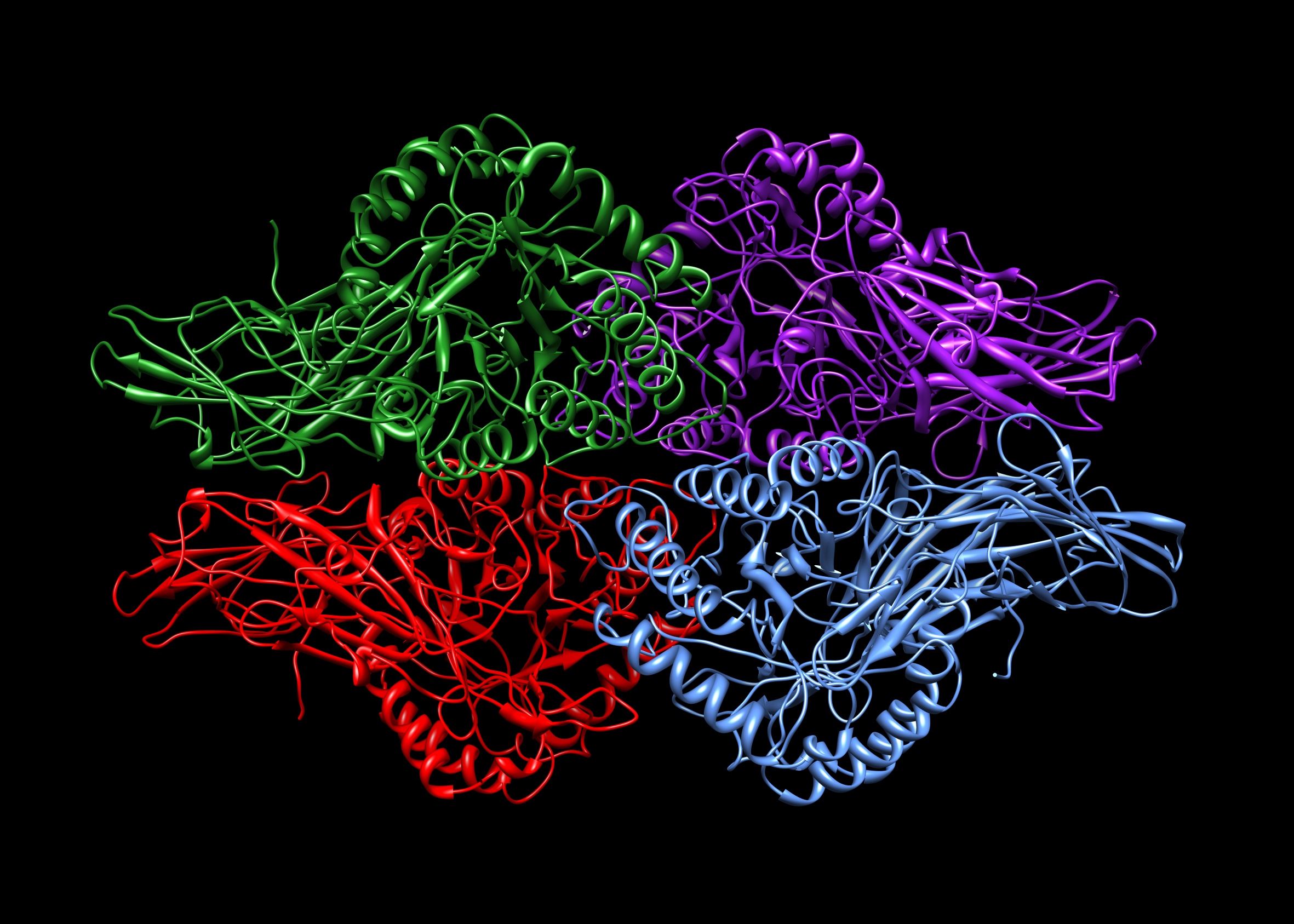
Heterotetramer
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homotetramer
A tetrameric protein is a protein with a quaternary structure of four subunits (tetrameric). Homotetramers have four identical subunits (such as glutathione S-transferase), and heterotetramers are complexes of different subunits. A tetramer can be assembled as dimer of dimers with two homodimer subunits (such as sorbitol dehydrogenase), or two heterodimer subunits (such as hemoglobin). Subunit interactions in tetramers The interactions between subunits forming a tetramer is primarily determined by non covalent interaction. Hydrophobic effects, hydrogen bonds and electrostatic interactions are the primary sources for this binding process between subunits. For homotetrameric proteins such as sorbitol dehydrogenase (SDH), the structure is believed to have evolved going from a monomeric to a dimeric and finally a tetrameric structure in evolution. The binding process in SDH and many other tetrameric enzymes can be described by the gain in free energy which can be determined ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Multiprotein Complex
A protein complex or multiprotein complex is a group of two or more associated polypeptide chains. Protein complexes are distinct from multidomain enzymes, in which multiple catalytic domains are found in a single polypeptide chain. Protein complexes are a form of quaternary structure. Proteins in a protein complex are linked by non-covalent protein–protein interactions. These complexes are a cornerstone of many (if not most) biological processes. The cell is seen to be composed of modular supramolecular complexes, each of which performs an independent, discrete biological function. Through proximity, the speed and selectivity of binding interactions between enzymatic complex and substrates can be vastly improved, leading to higher cellular efficiency. Many of the techniques used to enter cells and isolate proteins are inherently disruptive to such large complexes, complicating the task of determining the components of a complex. Examples of protein complexes include the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer Dimer Tetramer SDH
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization. Classification Chemistry classifies monomers by type, and two broad classes based on the type of polymer they form. By type: * natural vs synthetic, e.g. glycine vs caprolactam, respectively * polar vs nonpolar, e.g. vinyl acetate vs ethylene, respectively * cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively By type of polymer they form: * those that participate in condensation polymerization * those that participate in addition polymerization Differing stoichiometry causes each class to create its respective form of polymer. : The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the ratio of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotinylation
In biochemistry, biotinylation is the process of covalently attaching biotin to a protein, nucleic acid or other molecule. Biotinylation is rapid, specific and is unlikely to disturb the natural function of the molecule due to the small size of biotin (MW = 244.31 g/mol). Biotin binds to streptavidin and avidin with an extremely high affinity, fast on-rate, and high specificity, and these interactions are exploited in many areas of biotechnology to isolate biotinylated molecules of interest. Biotin-binding to streptavidin and avidin is resistant to extremes of heat, pH and proteolysis, making capture of biotinylated molecules possible in a wide variety of environments. Also, multiple biotin molecules can be Cross-link, conjugated to a protein of interest, which allows binding of multiple streptavidin, avidin or neutravidin protein molecules and increases the sensitivity of detection of the protein of interest. There is a large number of biotinylation reagents available that expl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer Assay
A tetramer assay (also known as a tetramer stain) is a procedure that uses tetrameric proteins to detect and quantify T cells that are specific for a given antigen within a blood sample. The tetramers used in the assay are made up of four major histocompatibility complex (MHC) molecules, which are found on the surface of most cells in the body. MHC molecules present peptides to T-cells as a way to communicate the presence of viruses, bacteria, cancerous mutations, or other antigens in a cell. If a T-cell's receptor matches the peptide being presented by an MHC molecule, an immune response is triggered. Thus, MHC tetramers that are bioengineered to present a specific peptide can be used to find T-cells with receptors that match that peptide. The tetramers are labeled with a fluorophore, allowing tetramer-bound T-cells to be analyzed with flow cytometry. Quantification and sorting of T-cells by flow cytometry enables researchers to investigate immune response to viral infection and vac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T Cell
T cells (also known as T lymphocytes) are an important part of the immune system and play a central role in the adaptive immune response. T cells can be distinguished from other lymphocytes by the presence of a T-cell receptor (TCR) on their cell surface receptor, cell surface. T cells are born from hematopoietic stem cells, found in the bone marrow. Developing T cells then migrate to the thymus gland to develop (or mature). T cells derive their name from the thymus. After migration to the thymus, the precursor cells mature into several distinct types of T cells. T cell differentiation also continues after they have left the thymus. Groups of specific, differentiated T cell subtypes have a variety of important functions in controlling and shaping the immune response. One of these functions is immune-mediated cell death, and it is carried out by two major subtypes: Cytotoxic T cell, CD8+ "killer" (cytotoxic) and T helper cell, CD4+ "helper" T cells. (These are named for the presen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD8+ T Cells
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pathogens such as viruses or bacteria, or cells that are damaged in other ways. Most cytotoxic T cells express T-cell receptors (TCRs) that can recognize a specific antigen. An antigen is a molecule capable of stimulating an immune response and is often produced by cancer cells, viruses, bacteria or intracellular signals. Antigens inside a cell are bound to class I MHC molecules, and brought to the surface of the cell by the class I MHC molecule, where they can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecule and the antigen, and the T cell destroys the cell. In order for the TCR to bind to the class I MHC molecule, the former must be accompanied by a glycoprotein ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Class I MHC
MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules (the other being MHC class II) and are found on the cell surface of all nucleated cells in the bodies of vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called ''cytosolic'' or ''endogenous pathway''. In humans, the HLAs corresponding to MHC class I are HLA-A, HLA-B, and HLA-C. Function Class I MHC molecules bind peptides generated mainly from the degradation of cytosolic proteins by the proteasome. The MHC I: peptide complex is then inserted via the endoplasmic reticulum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biotin
Biotin (also known as vitamin B7 or vitamin H) is one of the B vitamins. It is involved in a wide range of metabolic processes, both in humans and in other organisms, primarily related to the utilization of fats, carbohydrates, and amino acids. The name ''biotin'', borrowed from the German , derives from the Ancient Greek word (; 'life') and the suffix "-in" (a suffix used in chemistry usually to indicate 'forming'). Biotin appears as a white, needle-like crystalline solid. Chemical description Biotin is classified as a heterocyclic compound, with a sulfur-containing tetrahydrothiophene ring fused to a ureido group. A C5-carboxylic acid side chain is appended to the former ring. The ureido ring, containing the −N−CO−N− group, serves as the carbon dioxide carrier in carboxylation reactions. Biotin is a coenzyme for five carboxylase enzymes, which are involved in the catabolism of amino acids and fatty acids, synthesis of fatty acids, and gluconeogenesis. Biotinylat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β2M
β2 microglobulin (B2M) is a component of MHC class I molecules. MHC class I molecules have α1, α2, and α3 proteins which are present on all nucleated cells (excluding red blood cells). In humans, the β2 microglobulin protein is encoded by the ''B2M'' gene. Structure and function β2 microglobulin lies beside the α3 chain on the cell surface. Unlike α3, β2 has no transmembrane region. Directly above β2 (that is, further away from the cell) lies the α1 chain, which itself is next to the α2. β2 microglobulin associates not only with the alpha chain of MHC class I molecules, but also with class I-like molecules such as CD1 (5 genes in humans), MR1, the neonatal Fc receptor (FcRn), and Qa-1 (a form of alloantigen). Nevertheless, the β2 microglobulin gene is outside of the MHC (HLA) locus, on a different chromosome. An additional function is association with the HFE protein, together regulating the expression of hepcidin in the liver which targets the iron transporter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptavidin
Streptavidin is a 52 Atomic mass unit, kDa protein (tetramer) purified from the bacterium ''Streptomyces avidinii''. Streptavidin Homotetramer, homo-tetramers have an extraordinarily high affinity for biotin (also known as vitamin B7 or vitamin H). With a dissociation constant (Kd) on the order of ≈10−14 mol/L, the binding of biotin to streptavidin is one of the strongest non-covalent interactions known in nature. Streptavidin is used extensively in molecular biology and bionanotechnology due to the streptavidin-biotin complex's resistance to organic solvents, denaturants (e.g. guanidinium chloride), detergents (e.g. Sodium dodecyl sulfate, SDS, Triton X-100), proteolytic enzymes, and extremes of temperature and pH. Structure The crystal structure of streptavidin with biotin bound was reported by two groups in 1989. The structure was solved using multi wavelength anomalous diffraction by Hendrickson et al. at Columbia University and using multiple isomorphous replacemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |