|
Guidelines For Human Subject Research
Various organizations have created guidelines for human subject research for various kinds of research involving human subjects and for various situations. Instructions for the Directors of Clinics, Outpatient Clinics and Other Medical Facilities In 1892, Albert Ludwig Sigesmund Neisser, a German physician who is credited with the discovery of Neisseria gonorrhoeae, performed two sets of clinical trials attempting to find a method of prevention for syphilis. Neisser first inserted serum obtained from a single patient who had begun exhibiting the early signs of syphilis under the skin of four female patients,Vollmann, Jochen, and Rolf Winau. "Informed consent in human experimentation before the Nuremberg code." BMJ: British Medical Journal 313.7070 (1996): 1445. similar to the procedure for smallpox inoculation. Neisser did not obtain consent from these patients, but none of them developed the disease. Neisser then conducted the second set of trials on four prostitutes.Ligon, BL. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Subject Research
Human subject research is systematic, scientific investigation that can be either interventional (a "trial") or observational (no "test article") and involves human beings as research subjects, commonly known as test subjects. Human subject research can be either medical (clinical) research or non-medical (e.g., social science) research. Systematic investigation incorporates both the collection and analysis of data in order to answer a specific question. Medical human subject research often involves analysis of biological specimens, epidemiological and behavioral studies and medical chart review studies. (A specific, and especially heavily regulated, type of medical human subject research is the "clinical trial", in which drugs, vaccines and medical devices are evaluated.) On the other hand, human subject research in the social sciences often involves surveys which consist of questions to a particular group of people. Survey methodology includes questionnaires, interviews, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
US Congress
The United States Congress is the legislature, legislative branch of the federal government of the United States. It is a Bicameralism, bicameral legislature, including a Lower house, lower body, the United States House of Representatives, U.S. House of Representatives, and an Upper house, upper body, the United States Senate, U.S. Senate. They both meet in the United States Capitol in Washington, D.C. Members of Congress are chosen through direct election, though vacancies in the Senate may be filled by a Governor (United States), governor's appointment. Congress has a total of 535 voting members, a figure which includes 100 United States senators, senators and 435 List of current members of the United States House of Representatives, representatives; the House of Representatives has 6 additional Non-voting members of the United States House of Representatives, non-voting members. The vice president of the United States, as President of the Senate, has a vote in the Senate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Three Rs (animal Research)
The Three Rs (3Rs) are guiding principles for more ethical use of animals in product testing and scientific research. They were first described by W. M. S. Russell and R. L. Burch in 1959.Russell, W.M.S. and Burch, R.L., (1959). ''The Principles of Humane Experimental Technique'', Methuen, London.A digital version of the Principles may be accessed for free on thwebsiteof Johns Hopkins University's Center for Alternatives to Animal Testing (CAAT). The 3Rs are: #Replacement: methods which avoid the use of animals in research #Reduction: use of methods that enable researchers to minimise the number of animals necessary to obtain reliable and useful information. #Refinement: use of methods that alleviate or minimize potential pain, suffering, distress, or lasting harm and improve welfare for the animals used. The 3Rs have a broader scope than simply encouraging alternatives to animal testing, but aim to improve animal welfare and scientific quality where the use of animals cannot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patient And Public Involvement
Public involvement (or public and patient involvement, PPI) in medical research refers to the practice where people with health conditions (patients), carers and members of the public work together with researchers and influence what is researched and how. Involvement is not the same as participation which means taking part in research, for example taking a drug in a clinical trial. Definition Public involvement in medical research can be defined as research being carried out "''with''" or "''by''" members of the public rather than "''to''", "''about''" or "''for''" them. Through PPI patients, carers and people with lived experience work alongside researchers to influence and contribute to how research is designed and conducted. Members of the public involved in research are frequently referred to as public members or public contributors. Terminology Researchers and others use different terms to describe how they interact with the public, and this can vary across organisati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethics Committee
An ethics committee is a body responsible for ensuring that medical experimentation and human subject research are carried out in an ethical manner in accordance with national and international law. By jurisdiction European Union An ethics committee in the European Union is a body responsible for oversight of medical or human research studies in EU member states. Local terms for a European ethics committee include: * A Research Ethics Committee (REC) in the United Kingdom * A Medical Research Ethics Committee (MREC) in the Netherlands. * A Comité de Protection des Personnes (CPP) in France. United States In the United States, an ethics committee is usually known as an institutional review board (IRB) or research ethics board (REB) and is dedicated to overseeing the rights and well-being of research subjects participating in scientific studies in the US. Similarly in Canada, the committee is called a Research Ethics Board (REB). Australia In Australia, an ethics committee in med ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Informed Consent
Informed consent is an applied ethics principle that a person must have sufficient information and understanding before making decisions about accepting risk. Pertinent information may include risks and benefits of treatments, alternative treatments, the patient's role in treatment, and their Right to refuse medical treatment, right to refuse treatment. In most systems, healthcare providers have a legal and ethical responsibility to ensure that a patient's consent is informed. This principle applies more broadly than healthcare intervention, for example to conduct research, to disclose a person's medical information, or to participate in high risk sporting and recreational activities. Within the United States, definitions of informed consent vary, and the standard required is generally determined by the state. As of 2016, nearly half of the states adopted a reasonable patient standard, in which the informed consent process is viewed from the patient's perspective. These standards ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
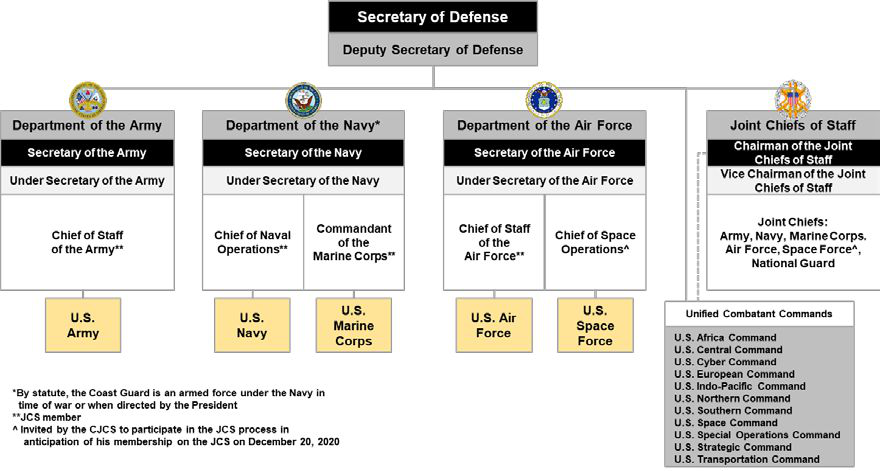
United States Department Of Defense
The United States Department of Defense (DoD, USDOD, or DOD) is an United States federal executive departments, executive department of the federal government of the United States, U.S. federal government charged with coordinating and supervising the six U.S. armed services: the United States Army, Army, United States Navy, Navy, United States Marine Corps, Marines, United States Air Force, Air Force, United States Space Force, Space Force, the United States Coast Guard, Coast Guard for some purposes, and related functions and agencies. As of November 2022, the department has over 1.4 million active-duty uniformed personnel in the six armed services. It also supervises over 778,000 National Guard (United States), National Guard and reservist personnel, and over 747,000 civilians, bringing the total to over 2.91 million employees. Headquartered at the Pentagon in Arlington County, Virginia, just outside Washington, D.C., the Department of Defense's stated mission is "to provid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychology
Psychology is the scientific study of mind and behavior. Its subject matter includes the behavior of humans and nonhumans, both consciousness, conscious and Unconscious mind, unconscious phenomena, and mental processes such as thoughts, feelings, and motivation, motives. Psychology is an academic discipline of immense scope, crossing the boundaries between the Natural science, natural and social sciences. Biological psychologists seek an understanding of the Emergence, emergent properties of brains, linking the discipline to neuroscience. As social scientists, psychologists aim to understand the behavior of individuals and groups.Hockenbury & Hockenbury. Psychology. Worth Publishers, 2010. A professional practitioner or researcher involved in the discipline is called a psychologist. Some psychologists can also be classified as Behavioural sciences, behavioral or Cognitive science, cognitive scientists. Some psychologists attempt to understand the role of mental functions in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
American Psychological Association
The American Psychological Association (APA) is the main professional organization of psychologists in the United States, and the largest psychological association in the world. It has over 170,000 members, including scientists, educators, clinicians, consultants, and students. It has 54 divisions, which function as interest groups for different subspecialties of psychology or topical areas. The APA has an annual budget of nearly $135 million. Profile The APA has task forces that issue policy statements on various matters of social importance, including abortion, human rights, the welfare of detainees, human trafficking, the rights of the Mental disorder, mentally ill, IQ testing, sexual orientation change efforts, and gender equality. Governance APA is a corporation chartered in Washington, D.C. APA's bylaws describe structural components that serve as a system of checks and balances to ensure democratic process. The organizational entities include: * APA President. The APA pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Office For Human Research Protections
The Office for Human Research Protections (OHRP) is a small office within the United States Department of Health and Human Services (DHHS), specifically the Office of the Assistant Secretary for Health in the Office of the Secretary of DHHS, that deals with ethical oversights in clinical research conducted by the department, mostly through the National Institutes of Health (NIH). The office's primary duty is the implementation of , a set of regulations for Institutional Review Boards (IRBs) that mirrors the U.S. Food and Drug Administration (FDA) regulation that covers clinical research conducted by pharmaceutical companies as well as other regulations under the guidance of the Federal Policy for the Protection of Human Subjects, which is also known as the "Common Rule". Institutions that conduct DHHS-sponsored research must have a "Federal-Wide Assurance" (FWA), an agreement with OHRP regarding ethical oversight. OHRP also provides education for IRBs, gives guidance on research ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Commission For The Protection Of Human Subjects Of Biomedical And Behavioral Research
National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research was the first public national body to shape bioethics policy in the United States. Formed in the aftermath of the Tuskegee Experiment scandal, the commission was created in 1974 as Title II of the National Research Act. It was part of the United States Department of Health, Education, and Welfare (DHEW) until 1978. Goals The commission had four goals that it needed to analyze: 1) the boundaries between biomedical and behavioral research and what the accepted and routine practices of medicine were 2) assessing the risks and benefits of the appropriateness of research involving human subjects 3) determining appropriate guidelines for how human subjects can be chosen for the participation in such research 4) defining what informed consent is in each research setting. Work The commission also had the task of making recommendations to the Secretary of Health, Education, and Welfare a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |