|
Gold–aluminium Intermetallic
Gold–aluminium intermetallic is a type of intermetallic compound of gold and aluminium that usually forms at contacts between the two metals. Gold–aluminium intermetallics have different properties from the individual metals, such as low conductivity and high melting point depending on their composition. Due to the difference of density between the metals and intermetallics, the growth of the intermetallic layers causes reduction in volume, and therefore creates gaps in the metal near the interface between gold and aluminium. The production of gaps lowers the strength of the metal compound, which can cause mechanical failure at the joint, fostering the problems that the intermetallics causes in metal compounds. In microelectronics, these properties can cause problems in wire bonding. The main compounds formed are usually Au5Al2 (white plague) and AuAl2 (purple plague), both of which form at high temperatures, then Au5Al2 and AuAl2 can further react with Au to form more stabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intermetallic
An intermetallic (also called intermetallic compound, intermetallic alloy, ordered intermetallic alloy, long-range-ordered alloy) is a type of metallic alloy that forms an ordered solid-state compound between two or more metallic elements. Intermetallics are generally hard and brittle, with good high-temperature mechanical properties. They can be classified as stoichiometric or nonstoichiometic. The term "intermetallic compounds" applied to solid phases has long been in use. However, Hume-Rothery argued that it misleads, suggesting a fixed stoichiometry and a clear decomposition into species. Definitions Research definition In 1967 defined intermetallic compounds as ''solid phases containing two or more metallic elements, with optionally one or more non-metallic elements, whose crystal structure differs from that of the other constituents''. This definition includes: * Electron (or Hume-Rothery) compounds * Size packing phases. e.g. Laves phases, Frank–Kasper phases and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microelectronics
Microelectronics is a subfield of electronics. As the name suggests, microelectronics relates to the study and manufacture (or microfabrication) of very small electronic designs and components. Usually, but not always, this means micrometre-scale or smaller. These devices are typically made from semiconductor materials. Many components of a normal electronic design are available in a microelectronic equivalent. These include transistors, capacitors, inductors, resistors, diodes and (naturally) insulators and conductors can all be found in microelectronic devices. Unique wiring techniques such as wire bonding are also often used in microelectronics because of the unusually small size of the components, leads and pads. This technique requires specialized equipment and is expensive. Digital integrated circuits (ICs) consist of billions of transistors, resistors, diodes, and capacitors. Analog circuits commonly contain resistors and capacitors as well. Inductors are u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
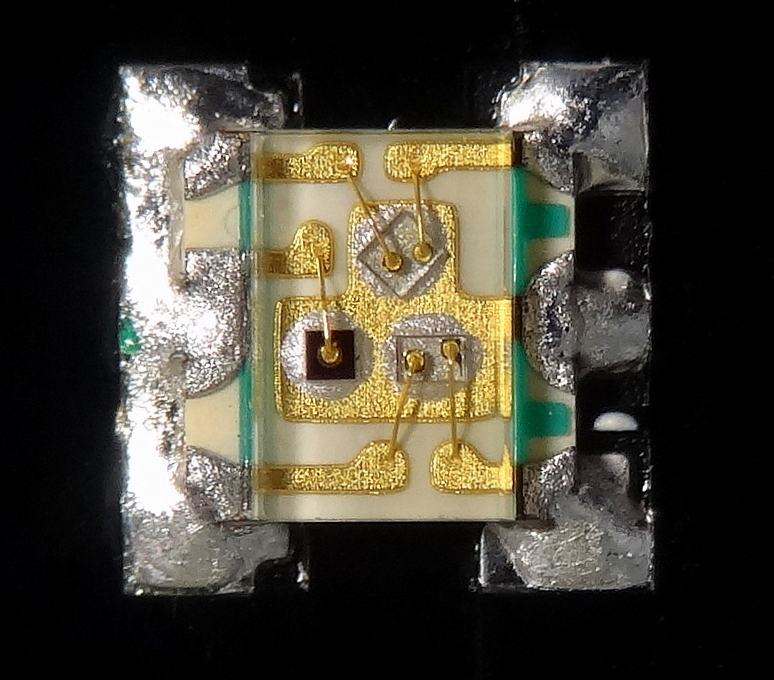
Wire Bonding
Wire bonding is a method of making interconnections between an integrated circuit (IC) or other semiconductor device and its packaging during semiconductor device fabrication. Wire bonding can also be used to connect an IC to other electronics or to connect from one printed circuit board (PCB) to another, although these are less common. Wire bonding is generally considered the most cost-effective and flexible interconnect technology and is used to assemble the vast majority of semiconductor packages. Wire bonding can be used at frequencies above 100 GHz. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical potential. It is possible to diffuse "uphill" from a region of lower concentration to a region of higher concentration, as in spinodal decomposition. Diffusion is a stochastic process due to the inherent randomness of the diffusing entity and can be used to model many real-life stochastic scenarios. Therefore, diffusion and the corresponding mathematical models are used in several fields beyond physics, such as statistics, probability theory, information theory, neural networks, finance, and marketing. The concept of diffusion is widely used in many fields, including physics (Molecular diffusion, particle diffusion), chemistry, biology, sociology, economics, statistics, data science, and finance (diffusion of people, ideas, data and price v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kirkendall Voiding
The Kirkendall effect is the motion of the interface between two metals that occurs due to the difference in diffusion rates of the metal atoms. The effect can be observed, for example, by placing insoluble markers at the interface between a pure metal and an alloy containing that metal, and heating to a temperature where atomic diffusion is reasonable for the given timescale; the boundary will move relative to the markers. This process was named after Ernest Kirkendall (1914–2005), assistant professor of chemical engineering at Wayne State University from 1941 to 1946. The paper describing the discovery of the effect was published in 1947. The Kirkendall effect has important practical consequences. One of these is the prevention or suppression of voids formed at the boundary interface in various kinds of alloy-to-metal bonding. These are referred to as Kirkendall voids. History The Kirkendall effect was discovered by Ernest Kirkendall and Alice Smigelskas in 1947, in the cours ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colored Gold
Colored Gold is the name given to any gold that has been treated using techniques to change its natural color. Pure gold is slightly reddish yellow in color, but colored gold can come in a variety of different colors by alloying it with different elements. Colored golds can be classified in three groups: * Alloys with silver and copper in various proportions, producing white, yellow, green and red golds. These are typically malleable alloys. * Intermetallic compounds, producing blue and purple golds, as well as other colors. These are typically brittle, but can be used as gems and inlays. * Surface treatments, such as oxide layers. Pure 100% (in practice, 99.9% or better) gold is 24 karat by definition, so all colored golds are less pure than this, commonly 18K (75%), 14K (58.5%), 10K (41.6%), or 9K (37.5%). Alloys White gold The word ''white'' covers a broad range of colors that borders or overlaps pale yellow, tinted brown, and even very pale rose. White gold is an alloy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Whiskers
Metal whiskering is a phenomenon that occurs in electrical devices when metals form long whisker-like projections over time. Tin whiskers were noticed and documented in the vacuum tube era of electronics early in the 20th century in equipment that used pure, or almost pure, tin solder in their production. It was noticed that small metal hairs or tendrils grew between metal solder pads, causing short circuits. Metal whiskers form in the presence of compressive stress. Germanium, zinc, cadmium, and even lead whiskers have been documented. Many techniques are used to mitigate the problem, including changes to the annealing process (heating and cooling), the addition of elements like copper and nickel, and the inclusion of conformal coatings. Traditionally, lead has been added to slow down whisker growth in tin-based solders. Following the Restriction of Hazardous Substances Directive (RoHS), the European Union banned the use of lead in most consumer electronic products from 2006 due ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen, or hydroxide. Rusting, the formation of red-orange iron oxides, is a well-known example of electrochemical corrosion. This type of corrosion typically produces oxides or salts of the original metal and results in a distinctive coloration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including mechanical strength, appearance, and permeability to liquids and ga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal, a group 11 element, and one of the noble metals. It is one of the least reactivity (chemistry), reactive chemical elements, being the second-lowest in the reactivity series. It is solid under standard temperature and pressure, standard conditions. Gold often occurs in free elemental (native state (metallurgy), native state), as gold nugget, nuggets or grains, in rock (geology), rocks, vein (geology), veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as in electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides). Gold is resistant to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |