|
Glycosynthases
The term glycosynthase refers to a class of proteins that have been engineered to catalyze the formation of a glycosidic bond. Glycosynthase are derived from glycosidase enzymes, which catalyze the hydrolysis of glycosidic bonds. They were traditionally formed from retaining glycosidase by mutating the active site nucleophilic amino acid (usually an aspartate or glutamate) to a small non-nucleophilic amino acid (usually alanine or glycine). More modern approaches use directed evolution to screen for amino acid substitutions that enhance glycosynthase activity. The first glycosynthase Two discoveries led to the development of glycosynthase enzymes. The first was that a change of the active site nucleophile of a glycosidase from a carboxylate to another amino acid resulted in a properly folded protein that had no hydrolase activity. The second discovery was that some glycosidase enzymes were able to catalyze the hydrolysis of glycosyl fluorides that had the incorrect anomeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosides
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzymatic, enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a ''C-glycoside'') glycosidic bond. Accord ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidase
In biochemistry, glycoside hydrolases (also called glycosidases or glycosyl hydrolases) are a class of enzymes which catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes, with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in ''N''-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosidase Transglycosylation Mechanism
In biochemistry, glycoside hydrolases (also called glycosidases or glycosyl hydrolases) are a class of enzymes which catalyze the hydrolysis of glycosidic bonds in complex sugars. They are extremely common enzymes, with roles in nature including degradation of biomass such as cellulose (cellulase), hemicellulose, and starch (amylase), in anti-bacterial defense strategies (e.g., lysozyme), in pathogenesis mechanisms (e.g., viral neuraminidases) and in normal cellular function (e.g., trimming mannosidases involved in ''N''-linked glycoprotein biosynthesis). Together with glycosyltransferases, glycosidases form the major catalytic machinery for the synthesis and breakage of glycosidic bonds. Occurrence and importance Glycoside hydrolases are found in essentially all domains of life. In prokaryotes, they are found both as intracellular and extracellular enzymes that are largely involved in nutrient acquisition. One of the important occurrences of glycoside hydrolases in bact ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anomeric
In carbohydrate chemistry, a pair of anomers () is a pair of near-identical stereoisomers or diastereomers that differ at only the anomeric carbon, the carbon atom that bears the aldehyde or ketone functional group in the sugar's open-chain form. However, in order for anomers to exist, the sugar must be in its cyclic form, since in open-chain form, the anomeric carbon atom is planar and thus achiral. More formally stated, then, an anomer is an epimer at the hemiacetal/hemiketal carbon atom in a cyclic saccharide. Anomerization is the process of conversion of one anomer to the other. As is typical for stereoisomeric compounds, different anomers have different physical properties, melting points and specific rotations. Nomenclature Every two anomers are designated alpha (α) or beta (β), according to the configurational relationship between the ''anomeric centre'' and the ''anomeric reference atom'', hence they are relative stereodescriptors. The anomeric centre in hemiace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exoglycosidase
Exoglycosidases are glycoside hydrolase enzymes that cleave the glycosidic linkage of a terminal monosaccharide in an oligosaccharide or polysaccharide. Because each residue is removed separately, a series of exoglycosidases, each one cleaving at a specific glycolic linkage, is needed. These exoglycosidases can be used to remove a terminal sugar residue, to determine the sequence of a glycan, or for modifying glycans on glycoprotein Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known a ...s.https://www.neb.com/products/glycobiology/exoglycosidases/exoglycosidases See also * Endoglycosidase References External links * Enzymes {{hydrolase-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosynthase Mechanism
The term glycosynthase refers to a class of proteins that have been engineered to catalyze the formation of a glycosidic bond. Glycosynthase are derived from glycosidase enzymes, which catalyze the hydrolysis of glycosidic bonds. They were traditionally formed from retaining glycosidase by mutating the active site nucleophilic amino acid (usually an aspartate or glutamate) to a small non-nucleophilic amino acid (usually alanine or glycine). More modern approaches use directed evolution to screen for amino acid substitutions that enhance glycosynthase activity. The first glycosynthase Two discoveries led to the development of glycosynthase enzymes. The first was that a change of the active site nucleophile of a glycosidase from a carboxylate to another amino acid resulted in a properly folded protein that had no hydrolase activity. The second discovery was that some glycosidase enzymes were able to catalyze the hydrolysis of glycosyl fluorides that had the incorrect anomeric co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcohol (chemistry)
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol, to complex, like sugar alcohols and cholesterol. The presence of an OH group strongly modifies the properties of Hydrocarbon, hydrocarbons, conferring Hydrophile, hydrophilic (water-loving) properties. The OH group provides a site at which many reactions can occur. History The flammable nature of the exhalations of wine was already known to ancient natural philosophers such as Aristotle (384–322 BCE), Theophrastus (–287 BCE), and Pliny the Elder (23/24–79 CE). However, this did not immediately lead to the isolation of alcohol, even despite the development of more advanced distillation techniques in second- and third-century Roman Egypt. An important recognition, first found in one of the writings attributed to Jabir ibn Hayyan, J� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leaving Group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving group'' is a less formal but more commonly used synonym of the term ''nucleofuge''; although IUPAC gives the term a broader definition. A species' ability to serve as a leaving group can affect whether a reaction is viable, as well as what mechanism the reaction takes. Leaving group ability depends strongly on context, but often correlates with ability to stabilize additional electron density from bond heterolysis. Common anionic leaving groups are , and halides and sulfonate esters such as tosylate (). Water (), alcohols (), and amines () are common neutral leaving groups, although they often require activating catalysts. Some moieties, such as hydride (H−) serve as leaving groups only extremely rarely. Nomenclature IUPAC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
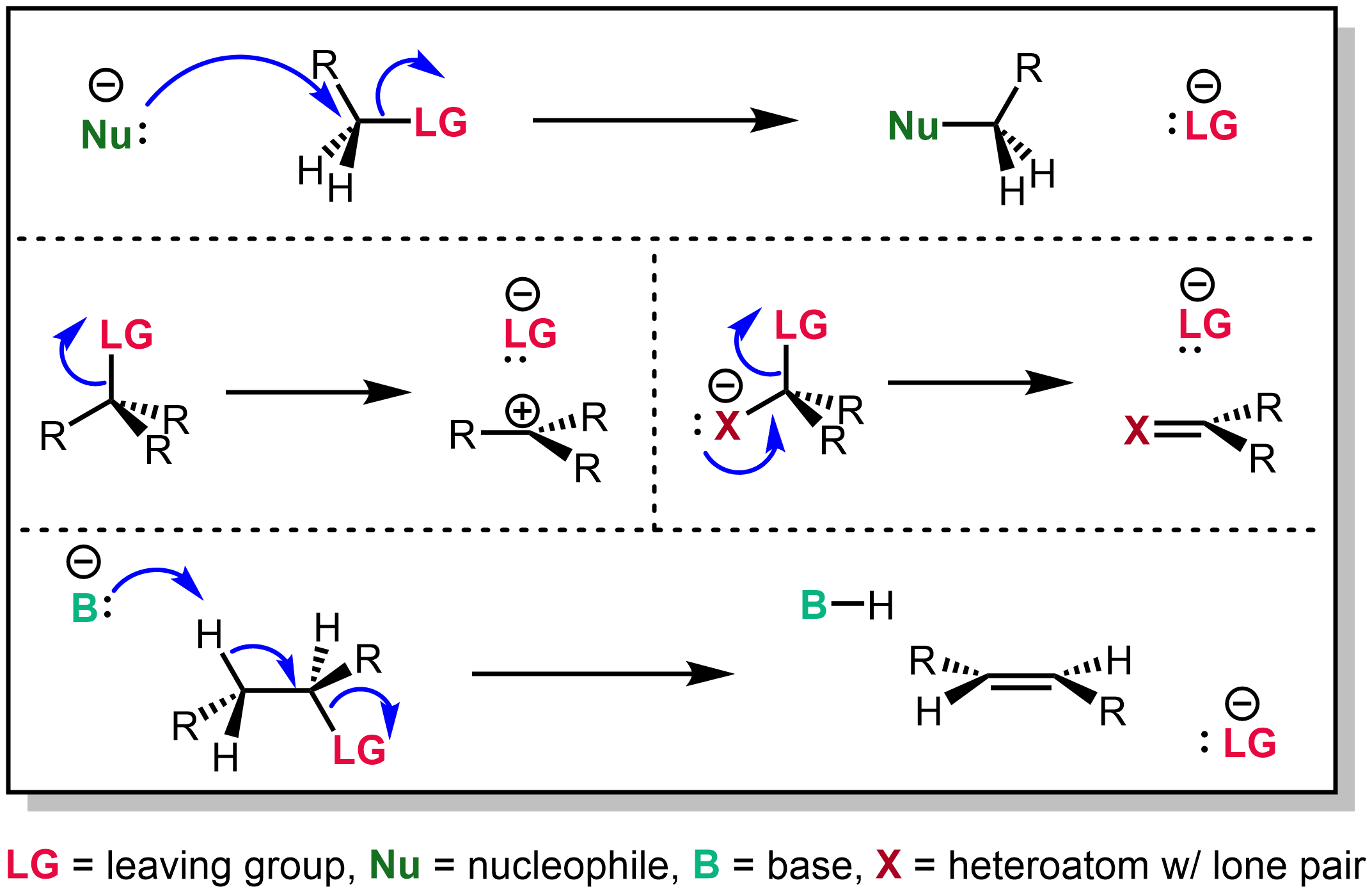
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trisaccharide
''Trisaccharides'' are oligosaccharides composed of three monosaccharides with two glycosidic bonds connecting them. Similar to the disaccharides, each glycosidic bond can be formed between any hydroxyl group on the component monosaccharides. Even if all three component sugars are the same (e.g., glucose), different bond combinations (regiochemistry) and stereochemistry Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ... (alpha- or beta-) result in trisaccharides that are diastereoisomers with different chemical and physical properties. Examples References External links * {{Carbohydrate-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutagenesis
Mutagenesis () is a process by which the genetic information of an organism is changed by the production of a mutation. It may occur spontaneously in nature, or as a result of exposure to mutagens. It can also be achieved experimentally using laboratory procedures. A mutagen is a mutation-causing agent, be it chemical or physical, which results in an increased rate of mutations in an organism's genetic code. In nature mutagenesis can lead to cancer and various heritable diseases, and it is also a driving force of evolution. Mutagenesis as a science was developed based on work done by Hermann Muller, Charlotte Auerbach and J. M. Robson in the first half of the 20th century. History DNA may be modified, either naturally or artificially, by a number of physical, chemical and biological agents, resulting in mutations. Hermann Muller found that "high temperatures" have the ability to mutate genes in the early 1920s, and in 1927, demonstrated a causal link to mutation upon experimen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |