|
Glycine Betaine Aldehyde
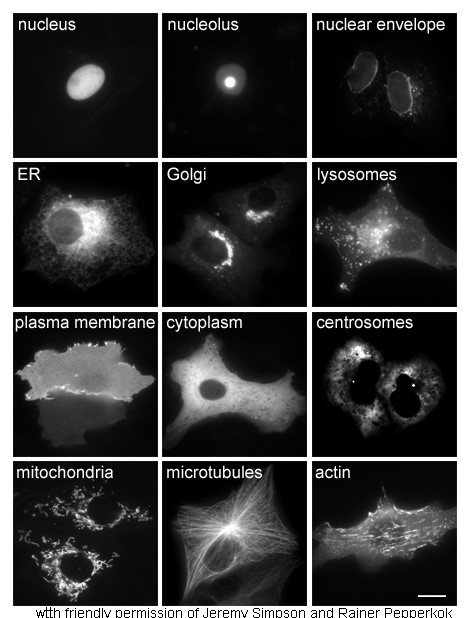
Glycine betaine aldehyde, often simply called betaine aldehyde, is an intermediate in the metabolism of glycine, serine and threonine. The human aldehyde dehydrogenase () stimulates the transformation of betaine aldehyde to glycine betaine. Betaine aldehyde is a substrate for choline dehydrogenase (mitochondrial). Chemical structure Glycine betaine aldehyde is a short chain aldehyde and quaternary ammonium compound. It can be considered a derivative of the amino acid glycine. Its chemical formula is C5H12NO+. Biological function Glycine betaine aldehyde is a component of glycine, serine and threonine metabolism. It also serves as an osmolyte. It can be found in cytoplasm and mitochondria within the kidney, neurons, and stratum corneum The stratum corneum (Latin language, Latin for 'horny layer') is the outermost layer of the epidermis (skin), epidermis. Consisting of dead tissue, it protects underlying tissue from infection, dehydration, chemicals and mechanical stress. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine disrupts the formation of alpha-helices in secondary protein structure. Its small side chain causes it to favor random coils instead. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into both hydrophilic and hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid. He originally called it "sugar of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Threonine
Threonine (symbol Thr or T) is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form when dissolved in water), a carboxyl group (which is in the deprotonated −COO− form when dissolved in water), and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as ''E. coli''. It is encoded by all the codons starting AC (ACU, ACC, ACA, and ACG). Threonine sidechains are often hydrogen bonded; the most common small motifs formed are based on interactions with serine: ST turns, ST motifs (often at the beginning of alpha helices) and ST staples (usually at the middle of alpha helices). Modifications The threonine residue is susceptible to numerous posttranslational modifications. The hydroxyl side-chain can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde Dehydrogenase
Aldehyde dehydrogenases () are a group of enzymes that catalyse the oxidation of aldehydes. They convert aldehydes (R–C(=O)) to carboxylic acids (R–C(=O)). The oxygen comes from a water molecule. To date, nineteen ALDH genes have been identified within the human genome. These genes participate in a wide variety of biological processes including the detoxification of exogenously and endogenously generated aldehydes. Function Aldehyde dehydrogenase is a polymorphic enzyme responsible for the oxidation of aldehydes to carboxylic acids. There are three different classes of these enzymes in mammals: class 1 (low ''K''m, cytosolic), class 2 (low ''K''m, mitochondrial), and class 3 (high ''K''m, such as those expressed in tumors, stomach, and cornea). In all three classes, constitutive and inducible forms exist. ALDH1 and ALDH2 are the most important enzymes for aldehyde oxidation, and both are tetrameric enzymes composed of 54 kDa subunits. These enzymes are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine Betaine
Trimethylglycine is an amino acid derivative with the formula . A colorless, water-soluble solid, it occurs in plants. Trimethylglycine is a zwitterion: the molecule contains both a quaternary ammonium group and a carboxylate group. Trimethylglycine was the first betaine discovered; originally it was simply called betaine because it was discovered in sugar beets (''Beta vulgaris'' subsp. ''vulgaris''). Several other betaines are now known. Medical uses Betaine, sold under the brand name Cystadane, is indicated for the adjunctive treatment of homocystinuria, involving deficiencies or defects in cystathionine beta-synthase (CBS), 5,10-methylene-tetrahydrofolate reductase (MTHFR), or cobalamin cofactor metabolism (cbl). The most common side effect is elevated levels of methionine in the blood. The EU has authorized the health claim that betaine "contributes to normal homocysteine metabolism.". Biological function Biosynthesis In most organisms, glycine betaine is biosynthesiz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Choline Dehydrogenase
In enzymology, a choline dehydrogenase () is an enzyme that catalyzes the chemical reaction :choline + acceptor \rightleftharpoons betaine aldehyde + reduced acceptor Thus, the two substrates of this enzyme are choline and acceptor, whereas its two products are betaine aldehyde and reduced acceptor. This enzyme belongs to the family of oxidoreductases, specifically those acting on the CH-OH group of donor with other acceptors. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivi ... of this enzyme class is choline:acceptor 1-oxidoreductase. Other names in common use include choline oxidase, choline-cytochrome c reductase, choline:(acceptor) oxidoreductase, and choline:(acceptor) 1-oxidoreductase. This enzyme participates in glycine, serine and threonine metabolism. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are a common motif in many chemicals important in technology and biology. Structure and bonding Aldehyde molecules have a central carbon atom that is connected by a double bond to oxygen, a single bond to hydrogen and another single bond to a third substituent, which is carbon or, in the case of formaldehyde, hydrogen. The central carbon is often described as being sp2- hybridized. The aldehyde group is somewhat polar. The bond length is about 120–122 picometers. Physical properties and characterization Aldehydes have properties that are diverse and that depend on the remainder of the molecule. Smaller aldehydes such as formaldehyde and acetaldehyde are solubl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Ammonium Compound
In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure , where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium, ammonium ion () and the primary, secondary, or tertiary ammonium cations, the Quaternary compound, quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds (called quaternary amines in oilfield parlance) are salt (chemistry), salts of quaternary ammonium cations. Polyquaternium, Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule. Quats are used in consumer applications including as antimicrobials (such as detergents and disinfectants), fabric softeners, and hair conditioners. As an antimicrobial, they are able to inactivate viral envelope, enveloped viruses (such as SARS-CoV-2). Quats tend to be gentler on surfaces th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks of proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their Structures#Biological, structures, and respond to their environments. The word ''metabolism'' can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as ''catabolic''—the ''breaking down'' of compounds (for example, of glucose to pyruvate by c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmolyte
Osmolytes are low-molecular-weight organic compounds that influence the properties of biological fluids. Osmolytes are a class of organic molecules that play a significant role in regulating osmotic pressure and maintaining cellular homeostasis in various organisms, particularly in response to environmental stressors. Their primary role is to maintain the integrity of cells by affecting the viscosity, melting point, and ionic strength of the aqueous solution. When a cell swells due to external osmotic pressure, membrane channels open and allow efflux of osmolytes carrying water, restoring normal cell volume. These molecules are involved in counteracting the effects of osmotic stress, which occurs when there are fluctuations in the concentration of solutes (such as ions and sugars) inside and outside cells. Osmolytes help cells adapt to changing osmotic conditions, thereby ensuring their survival and functionality. Osmolytes also interact with the constituents of the cell, e.g., the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol (a gel-like substance), the cell's internal sub-structures, and various cytoplasmic inclusions. In eukaryotes the cytoplasm also includes the nucleus, and other membrane-bound organelles.The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance, or cytoplasmic matrix, that remains after the exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, plant plasti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |