|
Gallium(III) Iodide
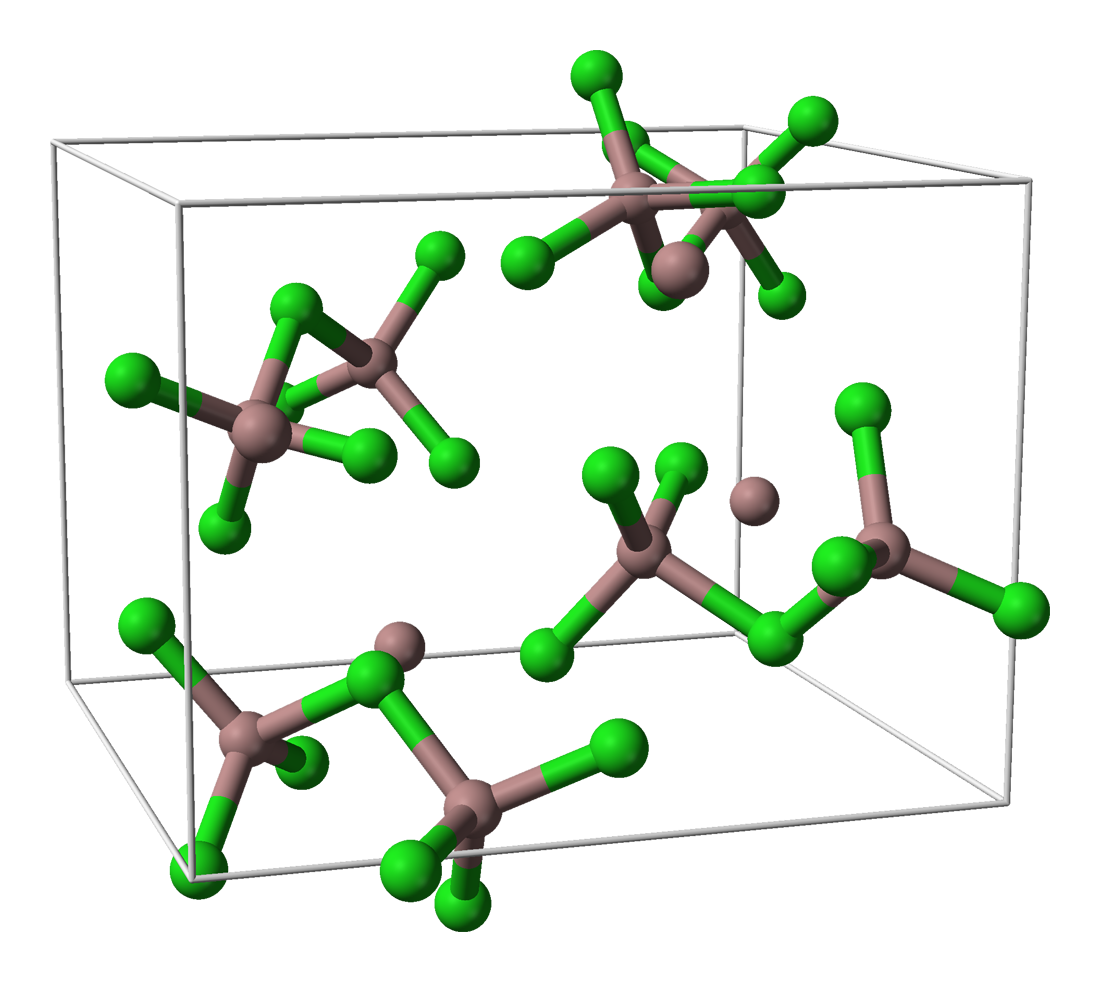
Gallium(III) iodide is the inorganic compound with the formula Ga I3. A yellow hygroscopic solid, it is the most common iodide of gallium. In the chemical vapor transport method of growing crystals of gallium arsenide uses iodine as the transport agent. In the solid state, it exists as the dimer Ga2I6, with a diborane structure. When vaporized, its forms GaI3 molecules of D3h symmetry where the Ga–I distance is 2.458 Angstroms The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874) .... Gallium triiodide can be reduced with gallium metal to give a green-colored gallium(I) iodide. The nature of this species is unclear, but it is useful for the preparation of gallium(I) and gallium(II) compounds. See also * Gallium halides References Cited sources * Iodides Gallium compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep Mantle (geology), mantle remain active areas of investigation. All allotropes (structurally different pure forms of an element) and some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, graphene, etc.), carbon monoxide , carbon dioxide , carbides, and salt (chemistry), salts of inorganic anions such as carbonates, cyanides, cyanates, thiocyanates, isothiocyanates, etc. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it cannot occur within life, living things. History ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include subscripts and superscripts. A chemical formula is not a chemical name since it does not contain any words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called '' empirical formulae'', which use letters and numbers indicating the numerical ''proportions'' of atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875, elemental gallium is a soft, silvery metal at standard temperature and pressure. In its liquid state, it becomes silvery white. If enough force is applied, solid gallium may fracture conchoidal fracture, conchoidally. Since its discovery in 1875, gallium has widely been used to make alloys with low melting points. It is also used in semiconductors, as a dopant in semiconductor substrates. The melting point of gallium, , is used as a temperature reference point. Gallium alloys are used in thermometers as a non-toxic and environmentally friendly alternative to Mercury (element), mercury, and can withstand higher temperatures than mercury. A melting point of , well below the freezing point of water, is claimed for the alloy galinstan (62–95% gallium, 5–22% indium, and 0–16% tin by weight), but that may be t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Vapor Transport
In chemistry, a chemical transport reaction describes a process for purification and crystallization of non-volatility (chemistry), volatile solids. The process is also responsible for certain aspects of mineral growth from the effluent of volcanoes. The technique is distinct from chemical vapor deposition, which usually entails decomposition of molecular precursors and which gives conformal coatings. The technique, which was popularized by Harald Schäfer, entails the reversible conversion of nonvolatile Chemical element, elements and chemical compounds into volatile derivatives. The volatile derivative migrates throughout a sealed reactor, typically a sealed and evacuated glass tube heated in a tube furnace. Because the tube is under a temperature gradient, the volatility (chemistry), volatile derivative reverts to the parent solid and the transport agent is released at the end opposite to which it originated (see next section). The transport agent is thus catalyst, catalyt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Arsenide
Gallium arsenide (GaAs) is a III-V direct band gap semiconductor with a Zincblende (crystal structure), zinc blende crystal structure. Gallium arsenide is used in the manufacture of devices such as microwave frequency integrated circuits, monolithic microwave integrated circuits, infrared light-emitting diodes, laser diodes, solar cells and optical windows. GaAs is often used as a substrate material for the epitaxial growth of other III-V semiconductors, including indium gallium arsenide, aluminum gallium arsenide and others. History Gallium arsenide was first synthesized and studied by Victor Goldschmidt in 1926 by passing arsenic vapors mixed with hydrogen over gallium(III) oxide at 600 °C. The semiconductor properties of GaAs and other Compound semiconductor, III-V compounds were patented by Heinrich Welker at Siemens-Schuckert in 1951 and described in a 1952 publication. Commercial production of its monocrystals commenced in 1954, and more studies followed in the 195 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diborane
Diborane(6), commonly known as diborane, is the chemical compound with the formula . It is a highly toxic, colorless, and pyrophoric gas with a repulsively sweet odor. Given its simple formula, borane is a fundamental boron compound. It has attracted wide attention for its electronic structure. Several of its derivatives are useful reagents. Structure and bonding The structure of diborane has D2h symmetry. Four hydrides are terminal, while two bridge between the boron centers. The lengths of the B–Hbridge bonds and the B–Hterminal bonds are 1.33 and 1.19 Å respectively. This difference in bond lengths reflects the difference in their strengths, the B–Hbridge bonds being relatively weaker. The weakness of the B–Hbridge compared to B–Hterminal bonds is indicated by their vibrational signatures in the infrared spectrum, being ≈2100 and 2500 cm−1 respectively. The model determined by molecular orbital theory describes the bonds between boron and the te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstroms
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström (). The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling ''angstrom''. The unit's symbol is Å, which is a letter of the Swedish alphabet, regardless of how the unit is spelled. However, "A" or "A.U." may be used in less formal contexts or typographically limited media. The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals, wavelengths of electromagnetic radiation, and dimensions of integrated circuit parts. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium(I) Iodide
Gallium monoiodide is an inorganic gallium compound with the formula GaI or Ga4I4. It is a pale green solid and mixed valent gallium compound, which can contain gallium in the 0, +1, +2, and +3 oxidation states. It is used as a pathway for many gallium-based products. Unlike the Gallium halides, gallium(I) halides first crystallographically characterized, gallium monoiodide has a more facile synthesis allowing a synthetic route to many low-valent gallium compounds. Synthesis In 1990, Malcolm Green (chemist), Malcolm Green synthesized gallium monoiodide by the Sonication, ultrasonication of liquid gallium metal with iodine in toluene yielding a pale green powder referred to as gallium monoiodide. The chemical composition of gallium monoiodide was not determined until the early to mid-2010s despite its simple synthesis. In 2012, the pale green gallium monoiodide was determined to be a combination of gallium metal and gallium(I,III) iodide, having the chemical composition [Ga0]2[Ga+] ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Halides
There are three sets of gallium halides, the trihalides where gallium has oxidation state +3, the intermediate halides containing gallium in oxidation states +1, +2 and +3 and some unstable monohalides, where gallium has oxidation state +1. Trihalides All four trihalides are known. They all contain gallium in the +3 oxidation state. Their proper names are gallium(III) fluoride, gallium(III) chloride, gallium(III) bromide and gallium(III) iodide. ;Gallium(III) fluoride, GaF3 :GaF3 is a white solid which sublimes before it melts, with an estimated melting point above 1000 °C. It contains 6 co-ordinate gallium atoms with a three-dimensional network of GaF6 octahedra sharing common corners. ;Gallium trichloride, GaCl3, Gallium tribromide, GaBr3 and gallium triiodide, GaI3 :These all have lower melting points than GaF3, (Gallium trichloride, GaCl3 mp 78 °C, GaBr3 mp 122 °C, GaI3 mp 212 °C) reflecting the fact that their structures all contain dimers with 4 coordin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Handbook Of Chemistry And Physics
The ''CRC Handbook of Chemistry and Physics'' is a comprehensive one-volume reference resource for science research. First published in 1914, it is currently () in its 105th edition, published in 2024. It is known colloquially among chemists as the "Rubber Bible", as CRC originally stood for "Chemical Rubber Company". As late as the 1962–1963 edition (3604 pages), the ''Handbook'' contained myriad information for every branch of science and engineering. Sections in that edition include: Mathematics, Properties and Physical Constants, Chemical Tables, Properties of Matter, Heat, Hygrometric and Barometric Tables, Sound, Quantities and Units, and Miscellaneous. ''Mathematical Tables from Handbook of Chemistry and Physics'' was originally published as a supplement to the handbook up to the 9th edition (1952); afterwards, the 10th edition (1956) was published separately as '' CRC Standard Mathematical Tables''. Earlier editions included sections such as "Antidotes of Poisons", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CRC Press
The CRC Press, LLC is an American publishing group that specializes in producing technical books. Many of their books relate to engineering, science and mathematics. Their scope also includes books on business, forensics and information technology. CRC Press is now a division of Taylor & Francis, itself a subsidiary of Informa. History The CRC Press was founded as the Chemical Rubber Company (CRC) in 1903 by brothers Arthur, Leo and Emanuel Friedman in Cleveland, Ohio, based on an earlier enterprise by Arthur, who had begun selling rubber laboratory aprons in 1900. The company gradually expanded to include sales of laboratory equipment to chemist A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...s. In 1913 the CRC offered a short (116-page) manual called the ''Rubber Handboo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |