|
Fructone
Fructone is the organic compound with the formula It is the ketal derived from the condensation of ethyl acetoacetate and ethylene glycol Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odo .... Also known as apple ketal and applinal, it has a fruity, apple-like smell with pineapple, strawberry, and woody aspects reminiscent of pine trees. It is a commercial fragrance. External links Fructone product page{Dead link, date=December 2019 , bot=InternetArchiveBot , fix-attempted=yes IFF including 3D chemical structure applet References Flavors Dioxolanes Carboxylate esters Sweet-smelling chemicals Perfume ingredients Ethyl esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketal
In organic chemistry, an acetal is a functional group with the connectivity . Here, the R groups can be organic fragments (a carbon atom, with arbitrary other atoms attached to that) or hydrogen, while the R' groups must be organic fragments not hydrogen. The two R' groups can be equivalent to each other (a "symmetric acetal") or not (a "mixed acetal"). Acetals are formed from and convertible to aldehydes or ketones and have the same oxidation state at the central carbon, but have substantially different chemical stability and reactivity as compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term ketal is sometimes used to identify structures associated with ketones (both R groups organic fragments rather than hydrogen) rather than aldehydes and, historically, the term acetal was used specifically for the aldehyde-related cases (having at least one hydrogen in place of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. Preparation At large scale, ethyl acetoacetate is industrially produced by treatment of diketene with ethanol. The small scale preparation of ethyl acetoacetate is a classic laboratory procedure. It involves Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol. : Reactions Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 8% of the total. The enol is moderately acidic. Thus ethyl acetoacetate behaves similarly to acetylacetone: : The resulting carbanion undergoes nucleophilic substitution. Ethyl acetoacetate is often used in the acetoacetic ester synthesis, comparable to diethyl malonate in the malonic ester synthesis or the Kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol ( IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste but is toxic in high concentrations. This molecule has been observed in outer space. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation : This reaction can be catalyzed by either acids or bases or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavors
Flavour or flavor is either the sensory perception of taste or smell, or a flavoring in food that produces such perception. Flavour or flavor may also refer to: Science * Flavors (programming language), an early object-oriented extension to Lisp *Flavour (particle physics) In particle physics, flavour or flavor refers to the ''species'' of an elementary particle. The Standard Model counts six flavours of quarks and six flavours of leptons. They are conventionally parameterized with ''flavour quantum numbers'' ..., a quantum number of elementary particles related to their weak interactions *Flavor of Linux, another term for any particular Linux distribution; by extension, "flavor" can be applied to any program or other computer code that exists in more than one current variant at the same time Film and TV * ''Flavors'' (film), romantic comedy concerning Asian-Indian immigrants in America * Flavour Network, is a Canadian TV channel with shows about food. Music Art ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
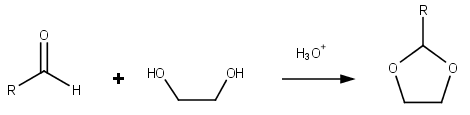
Dioxolanes
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran (THF) by replacement of the methylene group (CH2) at the 2-position with an oxygen atom. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-dioxolane is used as a solvent and as a comonomer in polyacetals. As a class of compounds Dioxolanes are a group of organic compounds containing the dioxolane ring. Dioxolanes can be prepared by acetalization of aldehydes and ketalization of ketones with ethylene glycol. (+)-''cis''-Dioxolane is the trivial name for which is a muscarinic acetylcholine receptor agonist. Protecting groups Organic compounds containing carbonyl groups sometimes need protection so that they do not undergo reactions during transformations of other functional groups that may be present. A variety of approaches to protection and deprotection ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylate Esters
In organic chemistry, a carboxylate is the conjugate base of a carboxylic acid, (or ). It is an anion, an ion with negative charge. Carboxylate salts are salts that have the general formula , where M is a metal and ''n'' is 1, 2,.... Carboxylate esters have the general formula (also written as ), where R and R′ are organic groups. Synthesis Carboxylate ions can be formed by deprotonation of carboxylic acids. Such acids typically have p''K''a of less than 5, meaning that they can be deprotonated by many bases, such as sodium hydroxide or sodium bicarbonate. : Resonance stabilization of the carboxylate ion Carboxylic acids easily dissociate into a carboxylate anion and a positively charged hydrogen ion (proton), much more readily than alcohols do (into an alkoxide ion and a proton), because the carboxylate ion is stabilized by resonance. The negative charge that is left after deprotonation of the carboxyl group is delocalized between the two electronegativ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfume Ingredients
Perfume (, ) is a mixture of fragrant essential oils or aroma compounds (fragrances), fixatives and solvents, usually in liquid form, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfumes can be defined as substances that emit and diffuse a pleasant and fragrant odor. They consist of artificial mixtures of aromatic chemicals and essential oils. The 1939 Nobel Laureate for Chemistry, Leopold Ružička stated in 1945 that "right from the earliest days of scientific chemistry up to the present time, perfumes have substantially contributed to the development of organic chemistry as regards methods, systematic classification, and theory." Ancient texts and archaeological excavations show the use of perfumes in some of the earliest human civilizations. Modern perfumery began in the late 19th century with the commercial synthesis of aroma compounds such as vanillin and coumarin, which allowed for the composition of perfumes with smells pre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |