|
Frontier Orbital
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontier orbitals'', such as in the frontier molecular orbital theory. Gap The energy difference between the HOMO and LUMO is ''the HOMO–LUMO gap''. Its size can be used to predict the strength and stability of transition metal complexes, as well as the colors they produce in solution. As a rule of thumb, the smaller a compound's HOMO–LUMO gap, the less stable the compound. Recent quantum‐chemical analyses of over 700 compounds demonstrated that terrestrial secondary metabolites exhibit HOMO–LUMO gaps on average about 2 eV narrower than organic molecules found in carbonaceous meteorites, and that combining gap width with hydrophilicity creates a robust discriminator between biotic and abiotic chemistries. This suggests that the HOMO–LUM ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecule HOMO-LUMO Diagram
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Dot
Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic in nanotechnology and materials science. When a quantum dot is illuminated by UV light, an electron in the quantum dot can be excited to a state of higher energy. In the case of a semiconducting quantum dot, this process corresponds to the transition of an electron from the valence band to the conduction band. The excited electron can drop back into the valence band releasing its energy as light. This light emission ( photoluminescence) is illustrated in the figure on the right. The color of that light depends on the energy difference between the discrete energy levels of the quantum dot in the conduction band and the valence band. In other words, a quantum dot can be defined as a structure on a semiconductor which is capable of confi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Koopmans' Theorem
Koopmans' theorem states that in closed-shell Hartree–Fock theory (HF), the first ionization energy of a molecular system is equal to the negative of the orbital energy of the highest occupied molecular orbital (HOMO). This theorem is named after Tjalling Koopmans, who published this result in 1934 for atoms. Koopmans' theorem is exact in the context of restricted Hartree–Fock theory if it is assumed that the orbitals of the ion are identical to those of the neutral molecule (the ''frozen orbital'' approximation). Ionization energies calculated this way are in qualitative agreement with experiment – the first ionization energy of small molecules is often calculated with an error of less than two electron volts. Therefore, the validity of Koopmans' theorem is intimately tied to the accuracy of the underlying Hartree–Fock wavefunction. The two main sources of error are orbital relaxation, which refers to the changes in the Fock operator and Hartree–Fock orbit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klopman–Salem Equation
In the theory of chemical reactivity, the Klopman–Salem equation describes the energetic change that occurs when two species approach each other in the course of a reaction and begin to interact, as their associated molecular orbitals begin to overlap with each other and atoms bearing partial charges begin to experience attractive or repulsive electrostatic forces. First described independently by Gilles Klopman and Lionel Salem in 1968, this relationship provides a mathematical basis for the key assumptions of frontier molecular orbital theory In chemistry, frontier molecular orbital theory is an application of molecular orbital theory describing HOMO and LUMO, HOMO–LUMO interactions. History In 1952, Kenichi Fukui published a paper in the ''Journal of Chemical Physics'' titled "A m ... (i.e., theory of HOMO–LUMO interactions) and hard soft acid base (HSAB) theory. Conceptually, it highlights the importance of considering both electrostatic interactions and orbital inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is , meaning that the 1s, 2s, and 2p subshells are occupied by two, two, and six electrons, respectively. Electronic configurations describe each electron as moving independently in an orbital, in an average field created by the nuclei and all the other electrons. Mathematically, configurations are described by Slater determinants or configuration state functions. According to the laws of quantum mechanics, a level of energy is associated with each electron configuration. In certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon. Knowledge of the electron configuration of different atoms is useful in understanding the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
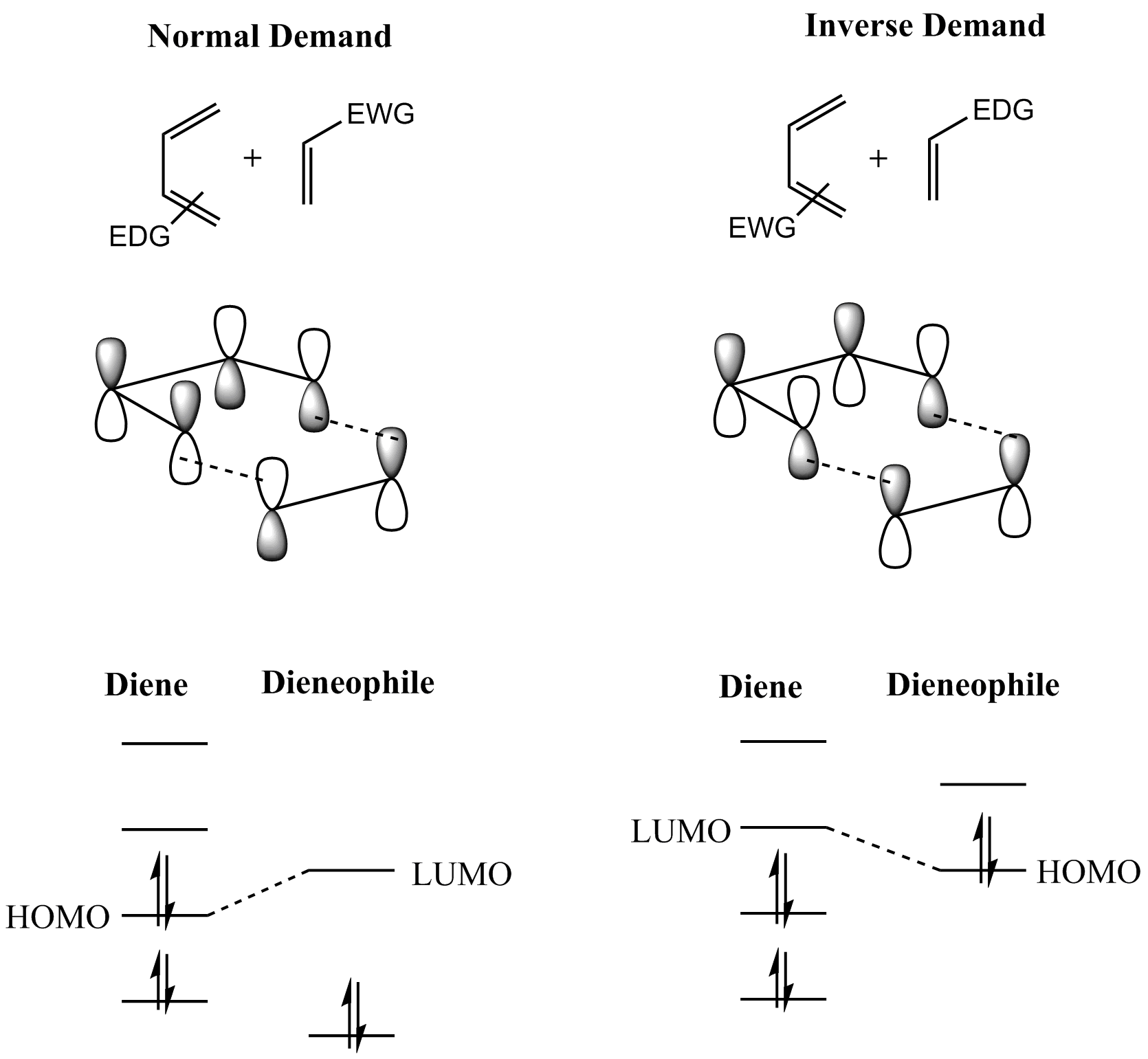
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition To Pi Ligands
Addition (usually signified by the plus symbol, +) is one of the four basic operations of arithmetic, the other three being subtraction, multiplication, and division. The addition of two whole numbers results in the total or '' sum'' of those values combined. For example, the adjacent image shows two columns of apples, one with three apples and the other with two apples, totaling to five apples. This observation is expressed as , which is read as "three plus two equals five". Besides counting items, addition can also be defined and executed without referring to concrete objects, using abstractions called numbers instead, such as integers, real numbers, and complex numbers. Addition belongs to arithmetic, a branch of mathematics. In algebra, another area of mathematics, addition can also be performed on abstract objects such as vectors, matrices, subspaces, and subgroups. Addition has several important properties. It is commutative, meaning that the order of the number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conduction Band
In solid-state physics, the valence band and conduction band are the bands closest to the Fermi level, and thus determine the electrical conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero temperature, while the conduction band is the lowest range of vacant electronic states. On a graph of the electronic band structure of a semiconducting material, the valence band is located below the Fermi level, while the conduction band is located above it. The distinction between the valence and conduction bands is meaningless in metals, because conduction occurs in one or more partially filled bands that take on the properties of both the valence and conduction bands. Band gap In semiconductors and insulators the two bands are separated by a band gap, while in conductors the bands overlap. A band gap is an energy range in a solid where no electron states can exist due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductors
A semiconductor is a material with electrical conductivity between that of a conductor and an insulator. Its conductivity can be modified by adding impurities (" doping") to its crystal structure. When two regions with different doping levels are present in the same crystal, they form a semiconductor junction. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for fabricating most electronic circuits. Semiconductor devices can display a range of different useful properties, such as passing current more easily in one ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CO2 HOMO
CO or variants may refer to: Chemistry * Carbon monoxide (CO), a colorless, odorless, and tasteless gas * Carbonyl group, composed of a carbon atom double-bonded to an oxygen atom: C=O * Cobalt, a chemical element, symbol Co Computing and telecommunications * .co (second-level domain), the Internet second-level domain meaning "commercial" * .co, the Internet country code top-level domain (ccTLD) for Colombia * Commitment ordering (CO), a concurrency control technique for databases * Telephone exchange, or central office (CO) Mathematics * Cofunction, or Co, in trigonometry * Cuboctahedron, a uniform polyhedron People * Nguyễn Hữu Có (1925–2012), Vietnamese general * Conrado Co (born 1940), Filipino badminton player * Alfredo Co (born 1949), Filipino Sinologist * Atoy Co (born 1951), Filipino actor and basketball coach * Leonard Co (1953–2010), Filipino botanist * Nando Có (born 1973), Bissau-Guinean footballer * Kenedy Có (born 1998), Bissau-Guinean ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |